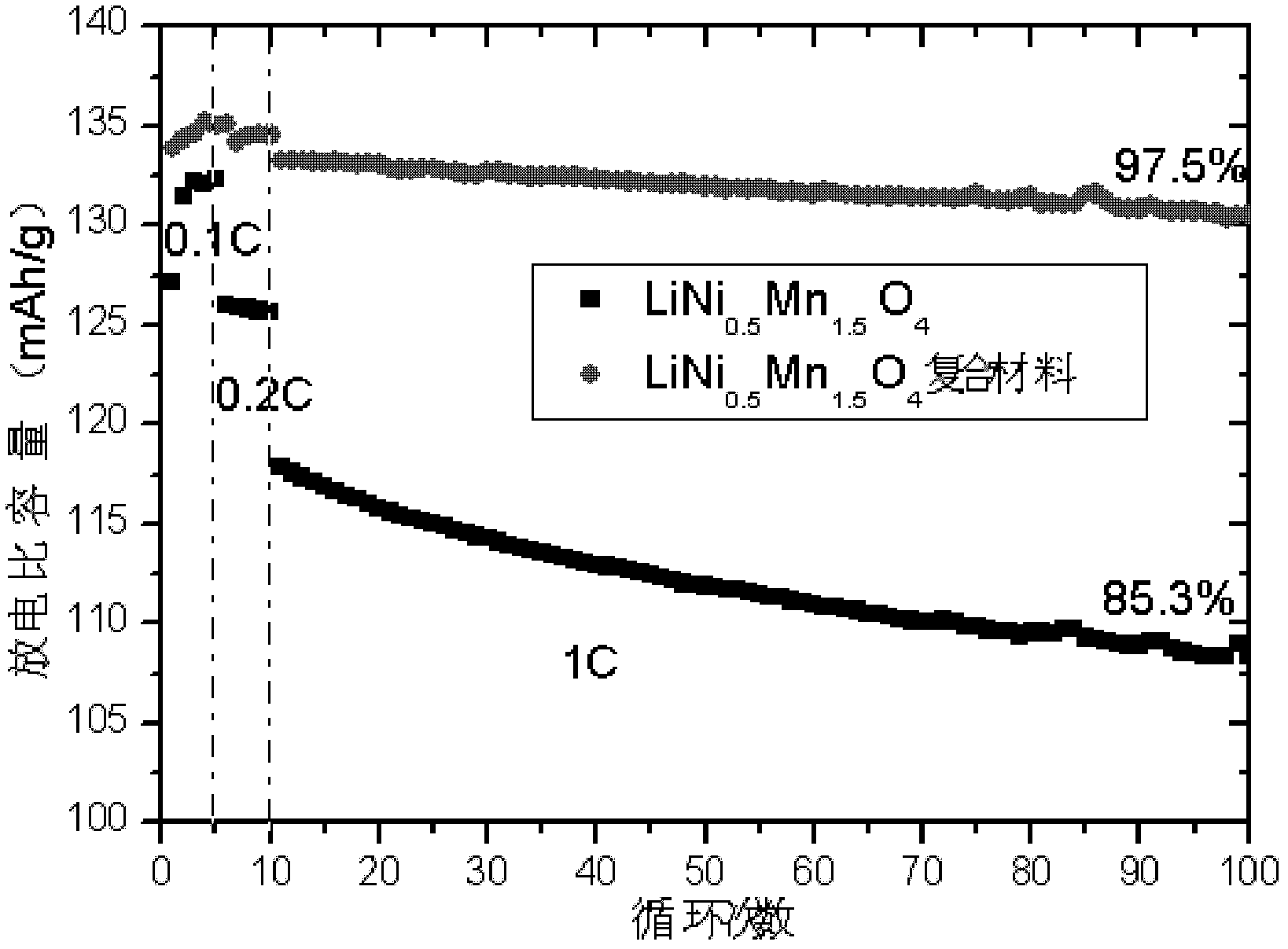
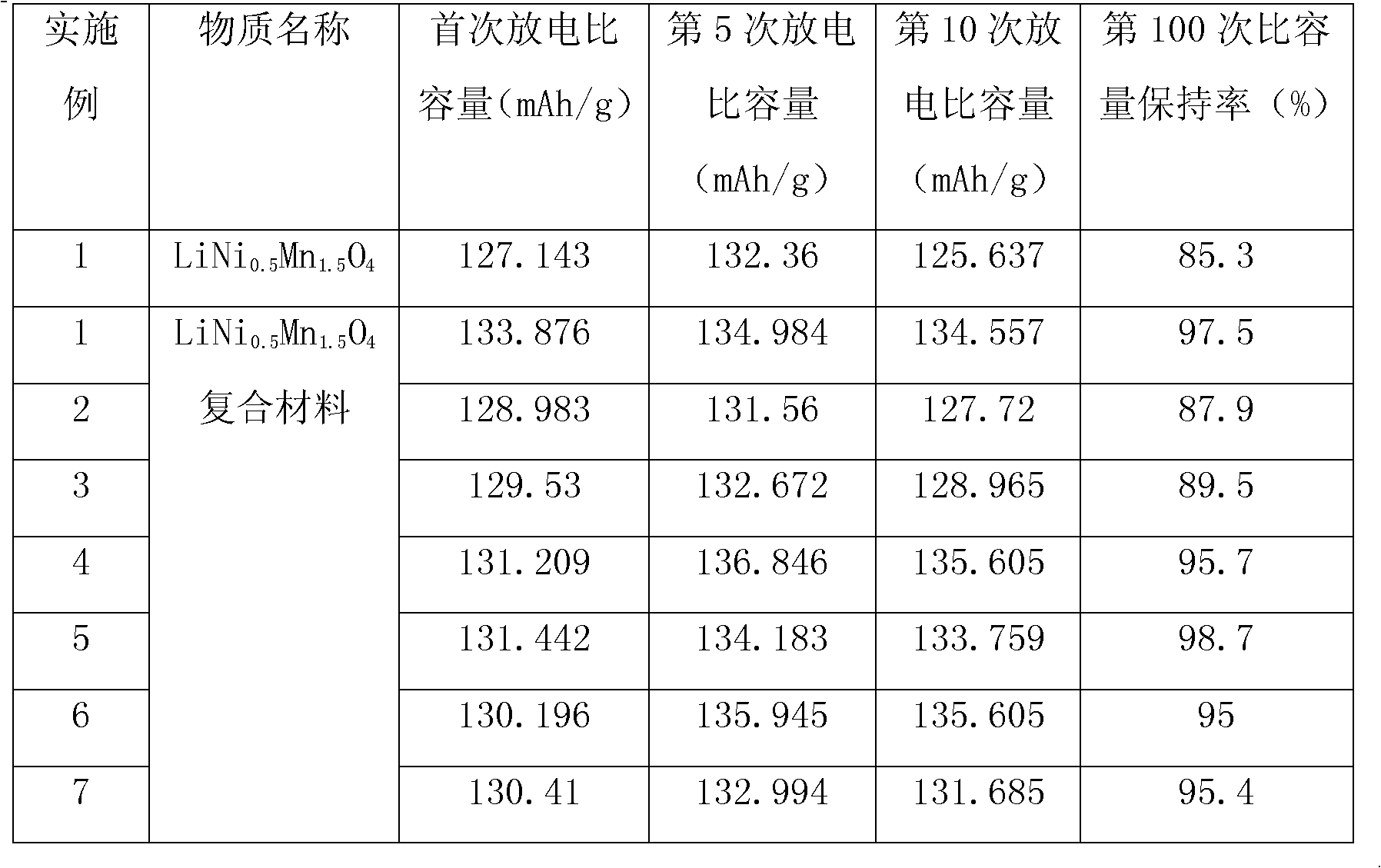
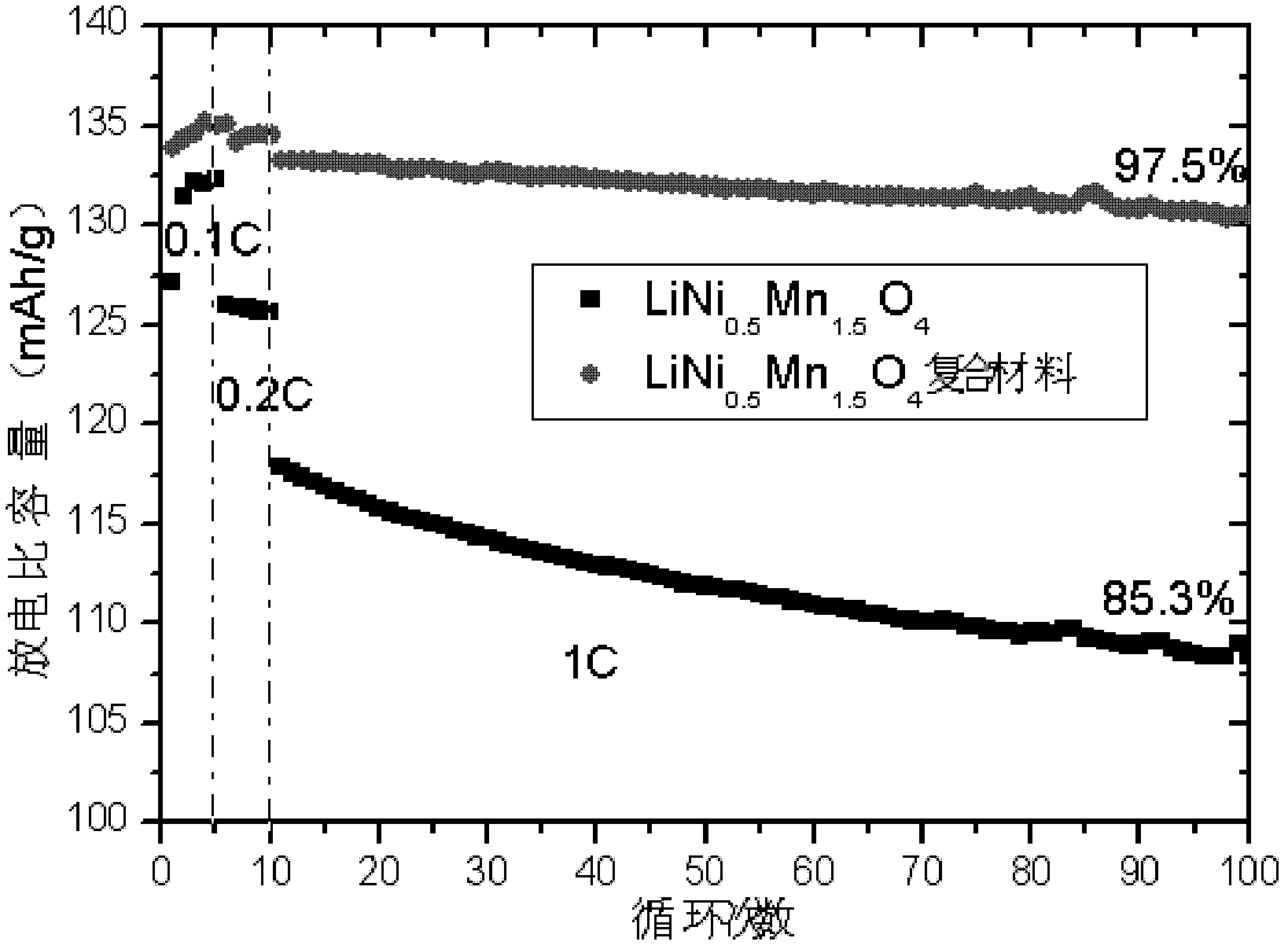
Lithium nickel manganese oxygen composite cathode material and preparation method thereof
A composite cathode material, lithium nickel manganese oxide technology, applied in battery electrodes, electrical components, circuits and other directions, can solve the problems of limited application, toxic pollution of the environment, unfavorable practical application, etc., to achieve the effect of improving cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Weigh 13.142g NiSO respectively 4 ·6H 2 O and 25.35gMnSO 4 ·H 2 O is prepared into a mixed aqueous solution. Under stirring, NaOH solution is added dropwise to the mixed solution, and the pH value of the mixed solution is controlled at about 10.3 to ensure Ni 2 +, Mn 2+ The precipitation is complete, after standing for 2 hours, suction filtration, washing three times, and drying at 110°C for 12 hours to obtain 17.85g Ni 0.5 Mn 1.5 (OH) 4 , Respectively weigh 3.6gNi 0.5 Mn 1.5 (OH) 4 And 0.739gLi 2 CO 3 , Ball milling to mix uniformly, then program temperature controlled air atmosphere sintering, heating to 500°C at a heating rate of 4°C / min, holding for 4 hours; then heating to 900°C at a heating rate of 5°C / min, calcination for 10 hours; then cooling at 5°C / min The speed drops to 600℃, annealing for 12h; finally, the temperature is naturally cooled to room temperature. Get 3.52g LiNi 0.5 Mn 1.5 O 4 .
[0039] 2. Weigh 6gLiNi separately 0.5 Mn 1.5 O 4 , 0.061g carbon bla...
Embodiment 2
[0042] 1. Preparation of LiNi 0.5 Mn 1.5 O 4 The scheme is the same as in Example 1
[0043] 2. Weigh 5gLiNi separately 0.5 Mn 1.5 O 4 , 0.048g acetylene black and 2.31g sucrose are added to the planetary ball mill, ball milled at 500r / min for 3h, the ball mill is evenly mixed, and sintered in an air atmosphere. The temperature rise rate is 5°C / min to 400°C, and the temperature is kept for 1.5 hours. The precursor body is carbonized to obtain lithium nickel manganese oxygen composite cathode material.
[0044] 3. The test methods of the composite cathode material assembled battery are the same as in Example 1
Embodiment 3
[0046] 1. Preparation of LiNi 0.5 Mn 1.5 O 4 The scheme is the same as in Example 1
[0047] 2. Weigh 8gLiNi separately 0.5 Mn 1.5 O 4 , 0.081g Super P, 0.472g polyvinyl alcohol were added to the planetary ball mill, ball milled at a speed of 350r / min for 7 hours, the ball mill was mixed uniformly, and sintered in an air atmosphere. The temperature was increased at a rate of 6°C / min to 500°C and kept for 1.5 hours. The precursor of the carbon source is carbonized to obtain lithium nickel manganese oxygen composite cathode material.
[0048] 3. The test methods of the composite cathode material assembled battery are the same as in Example 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com