Polymerizable photoinitiator and preparation method thereof
A polymerization photoinitiator and synthesis method technology, applied in the direction of organic chemistry, can solve the problems of easy hydrolysis, moisture and acid-base sensitivity, and achieve the effect of reducing migration and volatilization and increasing compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
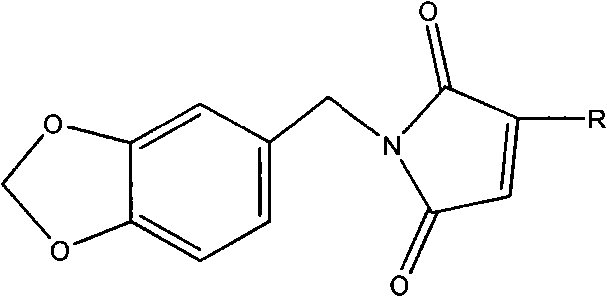
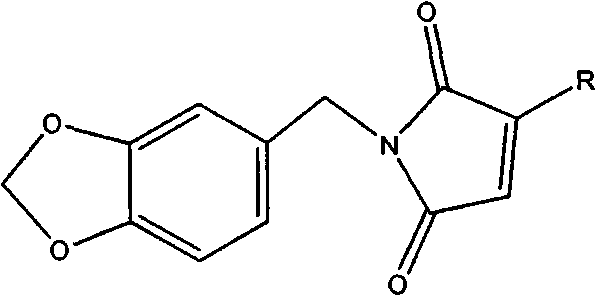
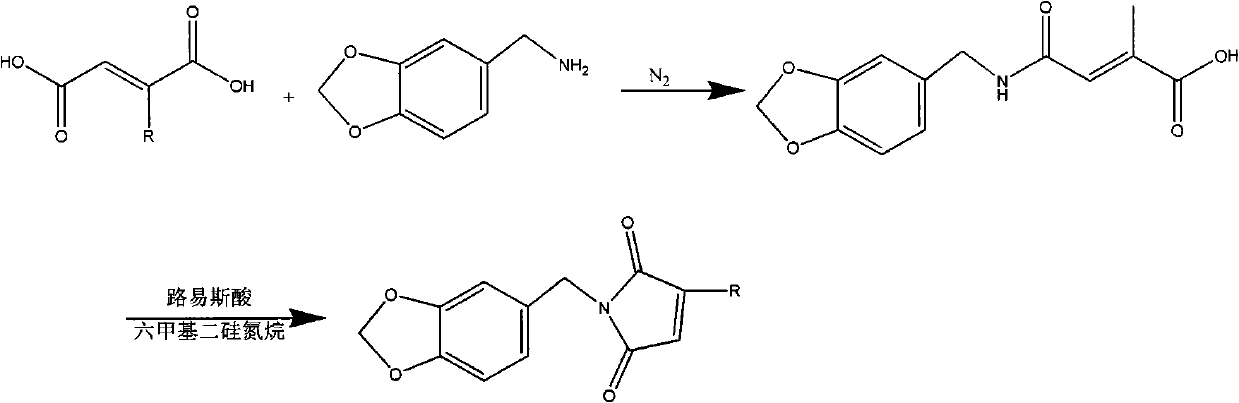
[0034]Dissolve 0.1 mole (13.71g) of 3,4-methylenedioxyaniline in 50mL of tetrahydrofuran and slowly drop into 0.1 mole (13.0g) of methyl fumaric acid in 60mL of tetrahydrofuran solution, pass through Nitrogen, when the color of the solution turns light yellow, add 0.16 moles (21.33g) of aluminum trichloride and continue to stir for 1h, then raise the temperature to 30°C, dissolve 0.2 moles (32.3g) of hexamethyldisilazane in 40mL Add dropwise into tetrahydrofuran, add dropwise for 3 hours, and reflux while adding dropwise. After the dropwise addition, cool to room temperature, filter the reactant, evaporate the solvent from the filtrate, and purify the residue with a silica gel column, the eluent is 70% cyclohexane / 30% ethyl acetate, evaporate the eluent under reduced pressure, vacuum After drying, the polymerizable photoinitiator N-3,4-methylenedioxyphenyl-2-methylmaleimide is obtained. The structure is shown in structural formula 4: 1H NMR (250MHz) in CDCl3: δ1.93ppm (3H, CH...
Embodiment 5
[0038] Dissolve 0.1 mole (13.71 g) of 3,4-methylenedioxyaniline in 50 mL of tetrahydrofuran and slowly drop into 0.3 mole (29.4 g) of maleic anhydride in 60 mL of tetrahydrofuran solution, and blow in argon, When the color of the solution turns light yellow, add 0.2 mol (27.2g) of zinc chloride and continue stirring for 0.5h, then raise the temperature to 45°C, and dissolve 0.1 mol (16.1g) of hexamethyldisilazane in 50mL of tetrahydrofuran Add dropwise, dropwise adding time 1.5h, reflux while adding dropwise. After the dropwise addition, cool to room temperature, filter the reactant, evaporate the solvent from the filtrate, and purify the residue with a silica gel column, the eluent is 70% cyclohexane / 30% ethyl acetate, evaporate the eluent under reduced pressure, vacuum After drying, the polymerizable photoinitiator N-3,4-methylenedioxyphenylmaleimide was obtained. The structure is shown in structural formula 1: 1H NMR (250MHz) in CDCl3: δ4.74-5.90ppm (4H, CH 2 ), 6.46-6.54...
Embodiment 6
[0042] Dissolve 0.1 mole (13.71g) of 3,4-methylenedioxyaniline in 40mL of tetrahydrofuran and slowly drop into 0.2 mole (26.0g) of methylene succinic acid in 60mL of tetrahydrofuran solution, blow nitrogen, When the color of the solution turns light yellow, add 0.12 moles (16.0g) of aluminum trichloride and continue to stir for 1.5h, then raise the temperature to 50°C, and dissolve 0.2 moles (32.2g) of hexamethyldisilazane in 90mL of tetrahydrofuran Add dropwise, add dropwise for 3 hours, and reflux while adding dropwise. After the dropwise addition, cool to room temperature, filter the reactant, evaporate the solvent from the filtrate, and purify the residue with a silica gel column, the eluent is 70% cyclohexane / 30% ethyl acetate, evaporate the eluent under reduced pressure, vacuum After drying, the polymerizable photoinitiator N-3,4-methylenedioxyphenyl-2-methylmaleimide is obtained. The structure is shown in structural formula 1: 1H NMR (250MHz) in CDCl3: δ1.93ppm (3H, CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com