Method for preparing (R)-phenyl glycol
A technology of phenylethylene glycol and styrene oxide, which is applied in the field of mung bean epoxide hydrolase catalyzed hydrolysis to prepare phenylethylene glycol, can solve the problem of non-enzymatic hydrolysis reaction of epoxy compounds, low reaction substrate concentration, Reduced ee value of the product and other issues, to achieve the effect of easy product, simple and easy control of the reaction process, and increased ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
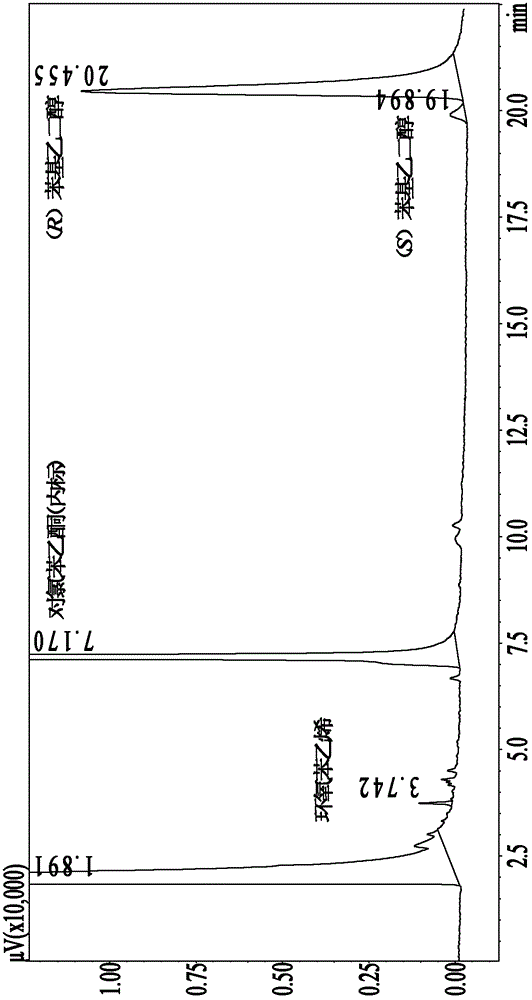
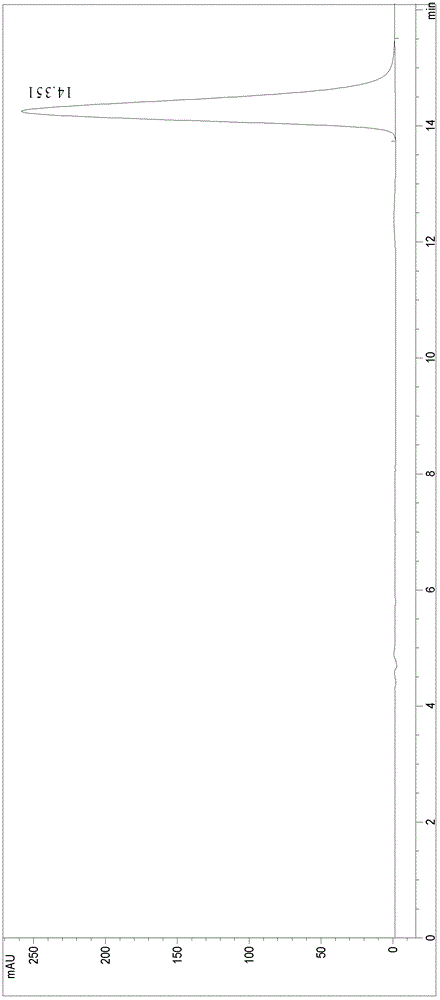
[0026] Add 1mL C to a 20mL Erlenmeyer flask with stopper 4 MIM·PF 6 and 6mL phosphate buffer (100mmol / L, pH 6.5) to form a biphasic system with a volume ratio of 1:6, then add 79.8μL epoxystyrene (100mmol / L) and 4.74U mung bean epoxide hydrolase Coarse enzyme powder is placed in a water bath shaker (220r / min) at 35°C for reaction. After reacting for 36 hours, the ionic liquid and phosphate buffer were separated by centrifugation, sodium chloride was added to the phosphate buffer to saturation, and then extracted with ethyl acetate, and the extract was rotary evaporated to remove ethyl acetate to obtain the product (R)-phenyl ethylene glycol. Product detection: two samples were taken from the buffer solution, and 2 times the volume of ethyl acetate (containing 7.7mmol / L p-chloroacetophenone) was added to one of them to extract the product for GC analysis of the ee value of the product; the other sample was used Methanol / water (30:70, v / v) was diluted 5 times, centrifuged (10...
Embodiment 2
[0028] Add 1mL C to a 20mL Erlenmeyer flask with stopper 4 MIM·PF 6 and 6mL phosphate buffer (100mmol / L, pH 6.5) to form a biphasic system with a volume ratio of 1:6, then add 15.96μL epoxystyrene (20mmol / L) and 4.74U mung bean epoxide hydrolase Coarse enzyme powder is placed in a water bath shaker (220r / min) at 35°C for reaction. After 12 h of reaction, (R)-phenylethylene glycol was obtained with an enantiomeric excess ee of 96.6% enantiomeric purity and a yield of 49.5%.
Embodiment 3
[0030] Add 1mL C to a 20mL Erlenmeyer flask with stopper 4 MIM·PF 6 and 6mL phosphate buffer (100mmol / L, pH 7.0) to form a biphasic system with a volume ratio of 1:6, then add 15.96μL epoxystyrene (20mmol / L) and 4.74U mung bean epoxide hydrolase Coarse enzyme powder is placed in a water bath shaker (220r / min) at 35°C for reaction. After 12 h of reaction, (R)-phenylethylene glycol was obtained with an enantiomeric excess ee of 96.3% enantiomeric purity and a yield of 49.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com