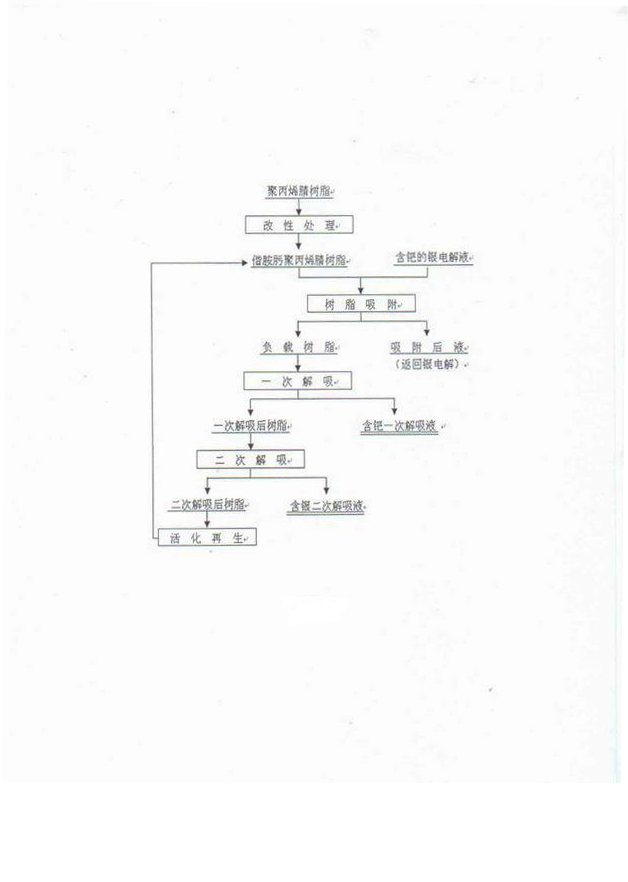
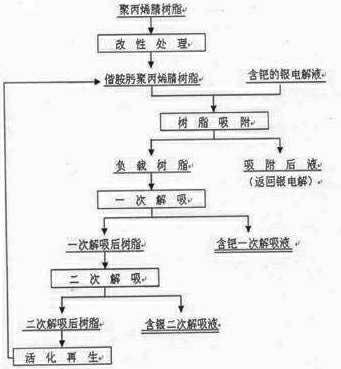
Separation method of palladium from silver electrolyte
An electrolyte and desorption technology, which is used in the improvement of process efficiency, photography technology, instruments, etc., can solve the problems that the electrolyte cannot be directly returned to the silver electrolysis, thiourea enters the silver electrolyte, and the separation process is not thorough enough, and the purification time is reached. The effect of short, high recycling rate and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0028] Commercially available polyacrylonitrile resin with a cross-linking degree of 10%; technical grade absolute ethanol, in which CH 3 CH 2 The OH content is 99.7%; analytically pure hydroxylammonium hydrochloride, of which HONH 2 The HCl content is 98.5%; analytically pure anhydrous sodium carbonate, of which Na 2 CO 3 The content is 99.8%; analytically pure thiourea, in which CS(NH 2 ) 2 The content is 99.0%; analytically pure nitric acid, of which HNO 3 The content is 65-68%; analytically pure hydrochloric acid, in which the HCl content is 36-38%; silver nitrate solution, in which Pd 500mg·L -1 , Ag 80g·L -1 , Cu 4g·L -1 , pH=0.05.
[0029] 10g of polyacrylonitrile resin, soaked in 100ml of absolute ethanol for 24h after wetting with water; prepare 900ml of 175 g·L -1 Hydrochloride solution of hydroxylammonium hydrochloride, adjust pH=7 with sodium carbonate, then add resin ethanol mixture (adding ethanol soaking solution together), modify it at 80°C for 8 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com