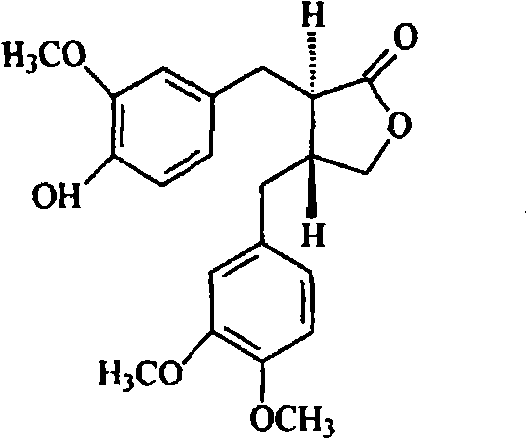
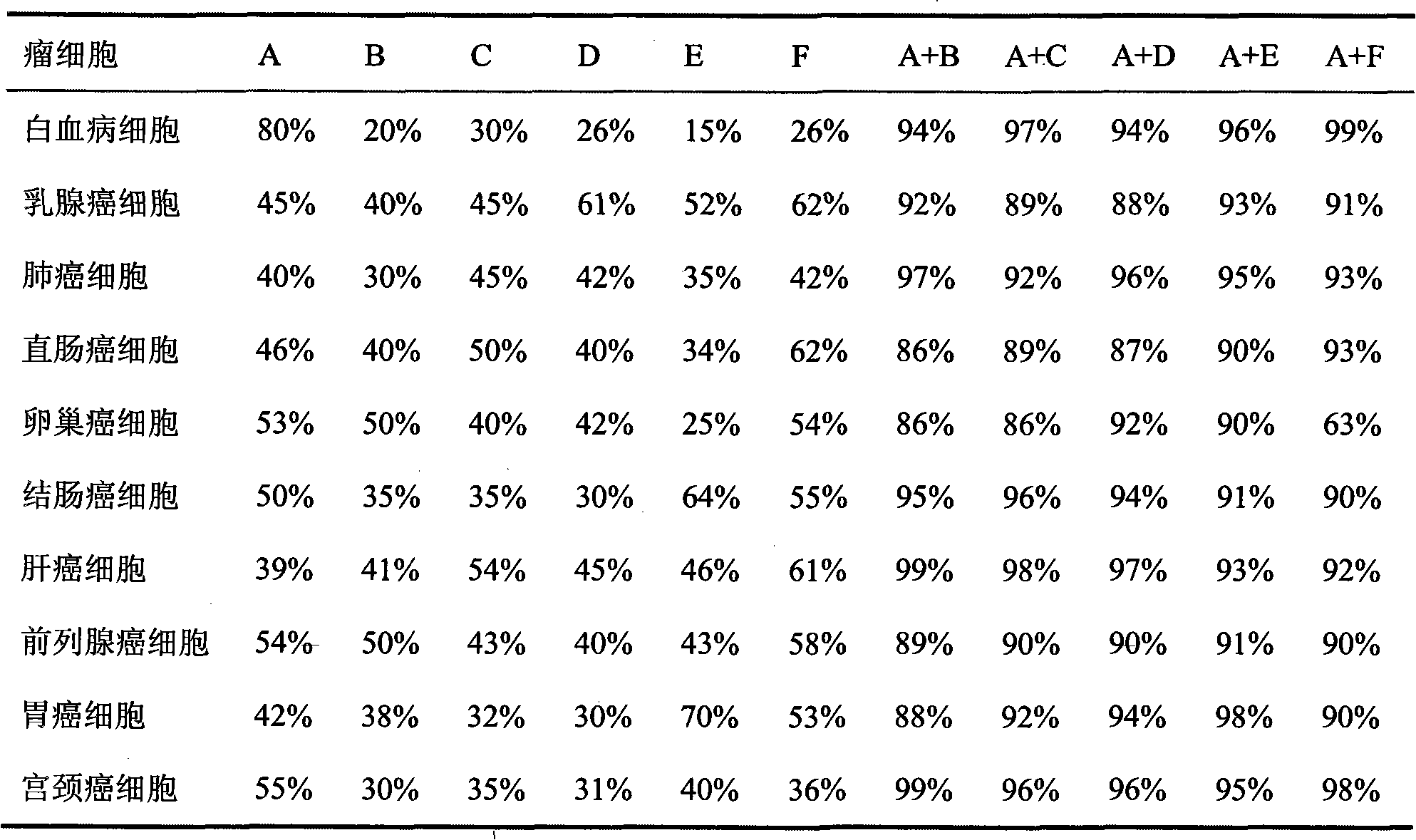
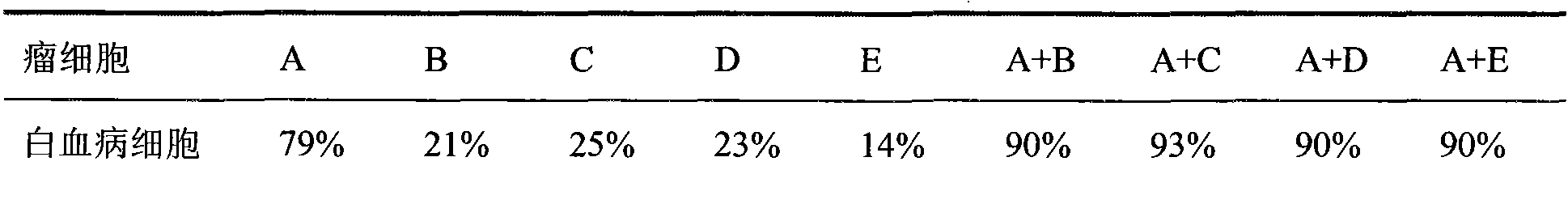
Tumor inhibiting medicine composition and purpose thereof
A technology of anti-tumor drugs and compositions, applied in the field of compositions containing arctigenin and pyrimidine nucleotide synthesis inhibitors, and anti-tumor drug compositions, which can solve the problems of long drug effect time, high treatment cost, and large side effects, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 arctigenin and capecitabine tablet
[0063] The tablet formulation of table 7 arctigenin and capecitabine composition
[0064]
[0065] Preparation process: Pass arctigenin and capecitabine through 80-mesh sieve respectively, weigh according to the prescription amount, mix evenly, then mix evenly with hydroxypropyl cyclodextrin in equal increments, pass through 60-mesh sieve, and then mix with Mix the rest of the excipients in the prescribed amount evenly, measure the content of the semi-finished product, calculate the weight of the tablet, and press the tablet.
Embodiment 2
[0066] Embodiment 2 Preparation of Arctigenin Gemcitabine Sustained Release Tablets
[0067] Table 8 Sustained-release tablet formulations of arctigenin and gemcitabine compositions
[0068]
[0069] Preparation process: Weigh arctigenin, gemcitabine, carbomer, and hydroxypropyl cellulose in prescription quantities and mix evenly. Take another appropriate amount of 8% starch slurry solution, add it to the mixed powder, mix evenly to make a soft material, pass through a 16-mesh sieve to granulate, and dry below 60°C. After the drying is completed, use an 18-mesh sieve to carry out granulation, sieve out the fine powder in the dry granules, mix with the sieved magnesium stearate, and then mix with the dry granules evenly, and press into tablets to obtain the product.
Embodiment 3
[0070] Embodiment 3 Preparation of arctigenin and tegafur microemulsion composition preparation
[0071] Table 9 arctigenin and tegafur microemulsion composition preparation 1
[0072]
[0073] Preparation process: Weigh 30 mg caprylic acid glyceride and 50 mg emulsifier OP, add to 20 mg propylene glycol, and mix well to obtain a suspension solution. Accurately weigh 25 mg of arctigenin and 0.25 mg of tegafur, add them to the suspension solution, and ultrasonically dissolve the arctigenin and tegafur to obtain the microemulsion concentrate of the arctigenin and tegafur composition. The microemulsion concentrate obtained above is diluted with water according to the weight ratio of 1:10-20 to a clear solution to obtain the microemulsion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com