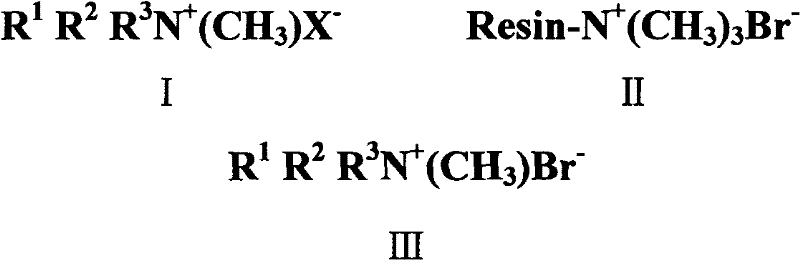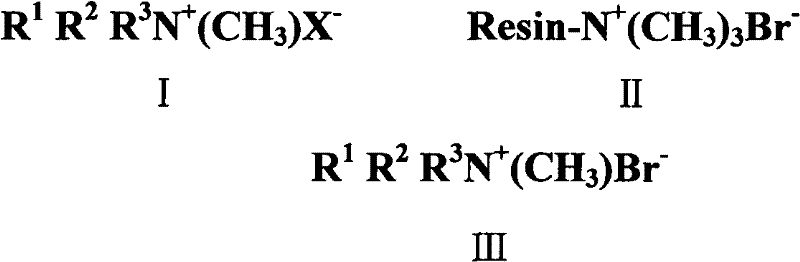Preparation method of quaternary ammonium salts of methyl ammonium bromide
A technology of methyl ammonium bromide and quaternary ammonium salt, which is applied in chemical instruments and methods, preparation of amino compounds from amines, organic chemistry, etc., can solve the problems of low exchange efficiency, narrow application range and high preparation cost, and achieves improved safety. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, the preparation of methyl diethyl hexadecyl ammonium bromide
[0042] A. Treatment of Brominated Strongly Basic Anion Exchange Resin
[0043] Add 400ml of resin to a glass chromatography column, add 1L of absolute ethanol to soak for 2h, wash the resin with deionized water until there is no alcohol smell, then soak in 1L of 2M hydrochloric acid for 2h, wash with deionized water until neutral, and wash with 2M hydrogen Wash with sodium oxide aqueous solution until there are no chloride ions (detection method: 2-3 drops of effluent, adjust the acidity with dilute nitric acid, add silver nitrate dropwise without precipitation), add deionized water to wash until neutral, and then wash the resin with saturated sodium bromide aqueous solution To pH = 7, add deionized water to wash until there is no bromide ion (detection method: take 2-3 drops of the effluent, add 1-2 drops of silver nitrate solution, no precipitation is formed);
[0044] B, the preparation of ...
Embodiment 2
[0047] Embodiment 2, the preparation of trimethyl (2-pentadecyl) eicosyl ammonium bromide
[0048] A. Treatment of Brominated Strongly Basic Anion Exchange Resin
[0049] Add about 220ml of resin used in Example 1 into a glass chromatography column, add 2L of absolute ethanol and soak for 5h, wash the resin with deionized water until there is no alcohol smell, and wash with 2M aqueous sodium hydroxide solution until there is no halogen ion (detection method: 2-3 drops of the effluent, adjust the acidity with dilute nitric acid, add dropwise silver nitrate (no precipitation), wash with deionized water until neutral, then wash the resin with saturated sodium bromide aqueous solution to pH = 7, wash with deionized water until no bromine Ion (detection method: take 2-3 drops of effluent, add 1-2 drops of silver nitrate solution, no precipitation is formed);
[0050] B, the preparation of trimethyl (2-pentadecyl) eicosyl ammonium bromide
[0051] At room temperature, 3.6g (5.2mmol)...
Embodiment 3、4
[0053] Example 3, 4,4'-[3α, 17β-bis(acetoxy)-5α-androstane-2β, 16β-dimethyl]-bis(1,1-dimethyl-piperazinium) Preparation of dibromide
[0054] A. Treatment of Brominated Strongly Basic Anion Exchange Resin
[0055] Processing method is with embodiment 1;
[0056] B, 4,4'-[3α,17β-bis(acetoxy)-5α-androstane-2β,16β-dimethyl]-bis(1,1-dimethyl-piperazinium)dibromide Compound preparation
[0057] At room temperature, 5.0 g (5.2 mmol) of 4,4'-[3α,17β-bis(acetyloxy)-5α-androstane-2β,16β-dimethyl]-bis(1,1-dimethyl Base-piperazinium) di-(p-toluenesulfonate) was dissolved in 300ml deionized water, took 220ml treated bromine-type strong basic anion exchange resin and packed it into a column, passed the above aqueous solution through the column, and washed with 300ml deionized water After removal, the effluent was collected and concentrated to dryness under reduced pressure at 60°C-80°C to obtain 3.7 g of white solid, melting point: 273°C-274°C (decomposition), yield 92.5% (HPLC>99.9%)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com