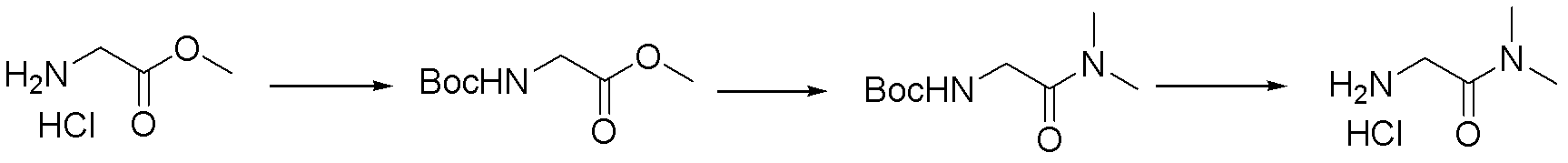
Method for preparing 2-amino-dimethyl acetamide hydrochloride
A technology of dimethylacetamide and hydrochloride, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of unsuitability for large-scale production, complicated operation procedures, and high cost of condensing agent raw materials. problems, to achieve the effect of cheap raw materials, stable process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A method for preparing 2-amino-N, N-dimethylacetamide hydrochloride, characterized in that the specific preparation steps are as follows:
[0023] (1) Amino protection: Add 55L (1.0eq) of 15% aqueous sodium carbonate solution, 50L (5mL / g) of dichloromethane, and 17.4kg (1.0eq) of di-tert-butyl dicarbonate into a 200L reactor in sequence, and the main raw material Glycine methyl ester hydrochloride 10kg, stirred and reacted at 0±2°C for 2 hours. After the reaction was completed, the liquids were separated, the aqueous phase was extracted with dichloromethane, the organic phase was combined with salt, washed with salt and then concentrated to obtain 14.5 kg of Boc-glycine methyl ester with a yield of 96.2% and a GC purity of 98.8%.
[0024] The NMR data of Boc-glycine methyl ester are as follows:
[0025] 1 H NMR (300MHZ, CDCl3), δ1.38 (H of tert-butyl 3 methyl groups), δ3.68 (H on methyl ester), δ3.90 (H on methylene), δ7.90 ( H on amino)
[0026] (2) Ammonolysis: Ad...
Embodiment 2
[0033] A method for preparing 2-amino-N, N-dimethylacetamide hydrochloride, characterized in that the specific preparation steps are as follows:
[0034](1) Amino group protection: Add 1kg of main raw materials glycine methyl ester hydrochloride, 15L of methyl tert-butyl ether (15mL / g), and 3.3kg of N-methylmorpholine (4.0eq) into a 50L reaction flask in sequence, and cool down To 30±2°C, add 3.5kg (2.0eq) of di-tert-butyl dicarbonate dropwise, and keep the reaction at 30±2°C for 1-2 hours after dropping. After the reaction is completed, concentrate until there is no solvent, add 6L of water, keep stirring for 1 to 2 hours, filter to obtain Boc-glycine methyl ester, and dry to obtain 1.44kg, the yield is 95.5%, and the GC purity is 98.9%;
[0035] The NMR data of Boc-glycine methyl ester are as follows:
[0036] 1 H NMR (300MHZ, CDCl3), δ1.42 (H of tert-butyl 3 methyl groups), δ3.56 (H on methyl ester), δ3.88 (H on methylene), δ7.87 ( H on the amino group).
[0037] (2) Am...
Embodiment 3
[0044] A method for preparing 2-amino-N, N-dimethylacetamide hydrochloride, characterized in that the specific preparation steps are as follows:
[0045] (1) Amino group protection: Add 25kg of the main raw materials glycine methyl ester hydrochloride, 200L (8mL / g) of tetrahydrofuran (8mL / g), 50.4kg (2.5eq) of triethylamine to a 500L reactor in sequence, cool down to 15±2°C, add dropwise 52.2kg (1.2eq) of di-tert-butyl dicarbonate, after dropping, keep it at 15±2℃ for 1~2h. After the reaction was completed, water was added, the liquid was separated, the organic phase was concentrated, the temperature was lowered, and 36 kg of Boc-glycine methyl ester was obtained by crystallization, with a yield of 95.5% and a GC purity of 99.4%.
[0046] The NMR data of Boc-glycine methyl ester are as follows:
[0047] 1 H NMR (300MHZ, CDCl3), δ1.40 (H of tert-butyl 3 methyl groups), δ3.67 (H on methyl ester), δ3.92 (H on methylene), δ7.95 ( H on the amino group).
[0048] (2) Ammonolysis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com