Methanesulfonic acid cinepazide crystal form III and preparation method thereof
A technology for cinepazide and cinepazide dihydrate, which is applied in the field of crystal forms of cinepazide mesylate and its preparation, and can solve the problems of increased cis isomer content, unstable chemical properties and the like , to achieve the effect of reducing cis isomers and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 cinepazide mesylate raw material
[0043] 1.1 Preparation of raw materials
[0044] According to the preparation method of the patent application "New medicinal salt of cinepazide and its preparation method" (application number: 200710096248.2), a self-colored crystalline powder is precipitated by cooling.
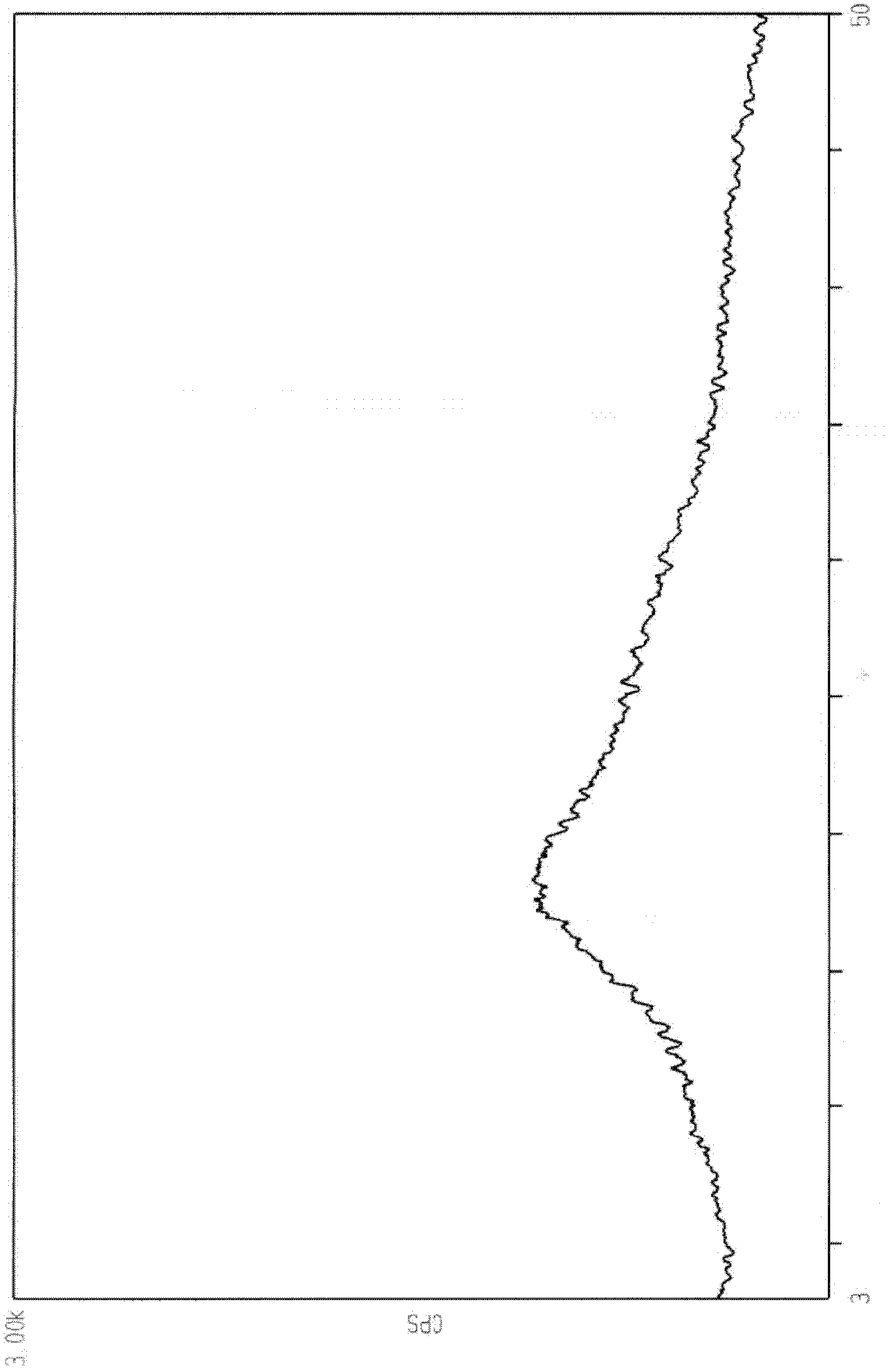
[0045] 1.2 XRD-powder diffraction pattern
[0046] See figure 1 , showing that the raw material prepared by the above-mentioned patent is an amorphous crystal powder.
[0047] 1.3 Solubility experiment
[0048] Solvents capable of dissolving cinepazide mesylate include: methanol, ethanol, n-propanol, isopropanol, n-butanol, isobutanol, sec-butanol, DMF, water, ethylene glycol monomethyl ether, dichloro Methane, chloroform, acetonitrile, nitromethane, DMSO. Among them, water, methanol, nitromethane, ethylene glycol monomethyl ether, and DMSO have excellent solubility, while others are average. Solvents that cannot dissolve cinepazide mesylate inclu...
Embodiment 2
[0049] Example 2 Cinepazide mesylate crystal form I
[0050] 2.1 Preparation method
[0051] Cinepazide mesylate in dichloromethane, chloroform, nitromethane, DMF, methanol / ether, dichloromethane / ether, methanol / methyl tert-butyl ether, DMF / ether, ethylene glycol monomethyl The crystals in ether / diethyl ether and ethylene glycol monomethyl ether / acetonitrile are all crystal forms I.
[0052] Method 1 (taking dichloromethane as an example): Add 0.5g of cinepazide mesylate to 25mL of dichloromethane under light-proof conditions, heat slightly to dissolve, stir for 1 hour, filter, and keep standing for three A few days later, the solvent was evaporated to dryness, and the solid was crushed, left to dry at room temperature for 6 hours, and samples were collected to obtain cinepazide mesylate crystal form I.
[0053] Method 2 (taking DMF / diethyl ether as an example): Add 0.7g of cinepazide mesylate to 20mL of DMF under dark conditions, dissolve, stir for 30min, add the solution d...
Embodiment 3
[0064] Example 3 Cinepazide mesylate crystal form II
[0065] 3.1 Preparation method
[0066] Cinepazide mesylate in methanol, ethanol, n-propanol, isopropanol, n-butanol, isobutanol, sec-butanol, acetonitrile, nitromethane / acetonitrile, DMSO / carbon tetrachloride / diethyl ether Crystallized as Form II.
[0067] Method 1 (taking methanol as an example): Add 2g of cinepazide mesylate to 5mL of methanol under light-shielding conditions, heat slightly to help dissolve, stir for 0.5h, filter, and keep it standing for about 23 days. Volatilized to dry up, collected samples, and obtained cinepazide mesylate crystal form II.
[0068] Method 2 (taking DMSO / carbon tetrachloride / diethyl ether as an example): Add 1 g of cinepazide mesylate to 15 mL of DMSO under dark conditions, dissolve, stir for 30 minutes, and add the solution dropwise to 150 mL of carbon tetrachloride In a mixed solvent with 100 mL of diethyl ether, white solid crystals were precipitated, filtered by suction, dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com