Stable method for preparing repaglinide compressed tablets
A stabilizer and mixing technology, applied in the field of preparation of repaglinide ordinary tablets, can solve the problems of difficult temperature control, increase of related substances, difference in content between particles and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
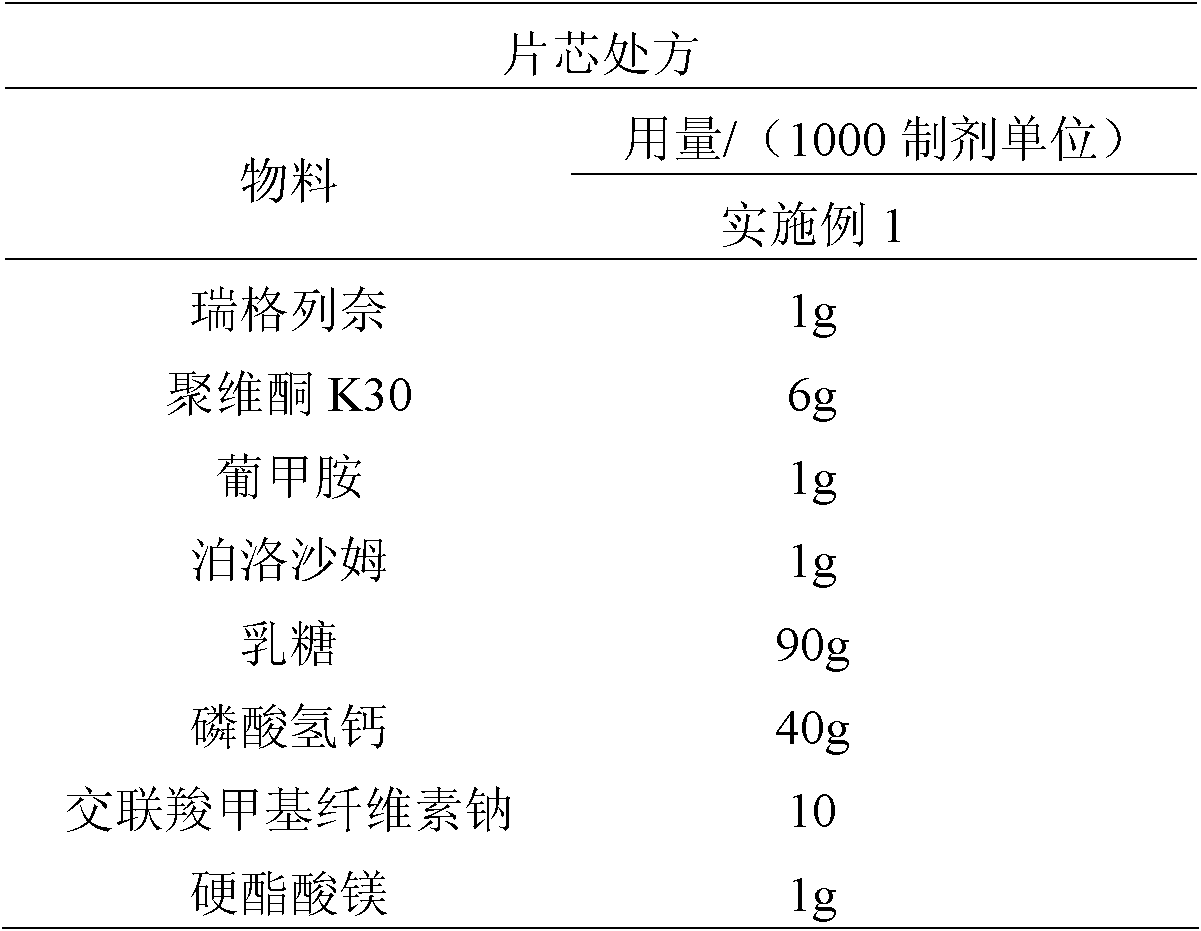
[0023] 1mg Repaglinide Ordinary Tablets
[0024]
[0025] Preparation:
[0026] Example 1: Povidone K30, meglumine, and poloxamer were dissolved in 70ml of purified water, repaglinide was dissolved in 50ml of ethanol, and repaglinide ethanol solution was added to the pure aqueous solution to dissolve completely. Using lactose and calcium hydrogen phosphate as fillers, the fluidized bed top spray granulation, the particle size is over 40 mesh. The granules are added with croscarmellose sodium and magnesium stearate, mixed evenly, and compressed into tablets.
Embodiment 2
[0037] 0.5mg Repaglinide Ordinary Tablets
[0038]
[0039] Preparation:
[0040] Example 2: Dissolve hydroxypropyl cellulose, meglumine, and sodium lauryl sulfate in 70ml of purified water, dissolve repaglinide in 50ml of ethanol, add repaglinide ethanol solution to the above-mentioned pure aqueous solution Dissolve completely. Using mannitol and calcium hydrogen phosphate as fillers, fluidized bed top spray granulation, the particle size is over 40 mesh. The granules are added with croscarmellose sodium and magnesium stearate, mixed evenly, and compressed into tablets.
Embodiment 3
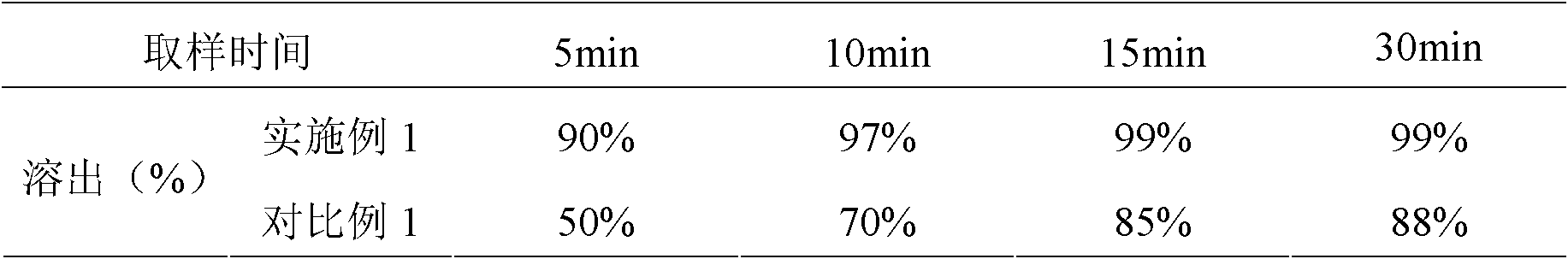
[0050] After 1.0 mg and 0.5 mg of repaglinide tablets of Examples 1 and 2 and Comparative Examples 1 and 2 were placed under certain conditions for 10 days, HPLC was used to analyze the content of repaglinide and related substances.
[0051] 1) High temperature test
[0052] The test sample was placed in a suitable clean container with the open top open, and placed at a temperature of 60° C. for 10 days, and the content of repaglinide in the test sample was detected. The results are shown in Table 5.
[0053] Table 5 High temperature (60°C) comparative test results
[0054]
[0055]
[0056] 2) High humidity test
[0057] Put the test product in a closed container with constant humidity, and place it at 25°C and 75.0% relative humidity for 10 days.
[0058] Detect the content and related substances of repaglinide in the test product, and the results are shown in Table 6.
[0059] Table 6 High humidity (RH75.0%) comparative test results
[0060]
[0061] It can be kn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com