Medicinal composition of methanesulfonic acid arbidol oral solid preparation
A solid preparation and composition technology, applied in the field of oral pharmaceutical preparations, can solve the problems of slow dissolution of preparations and affect absorption, and achieve the effects of good dispersion state, convenient taking and reasonable formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
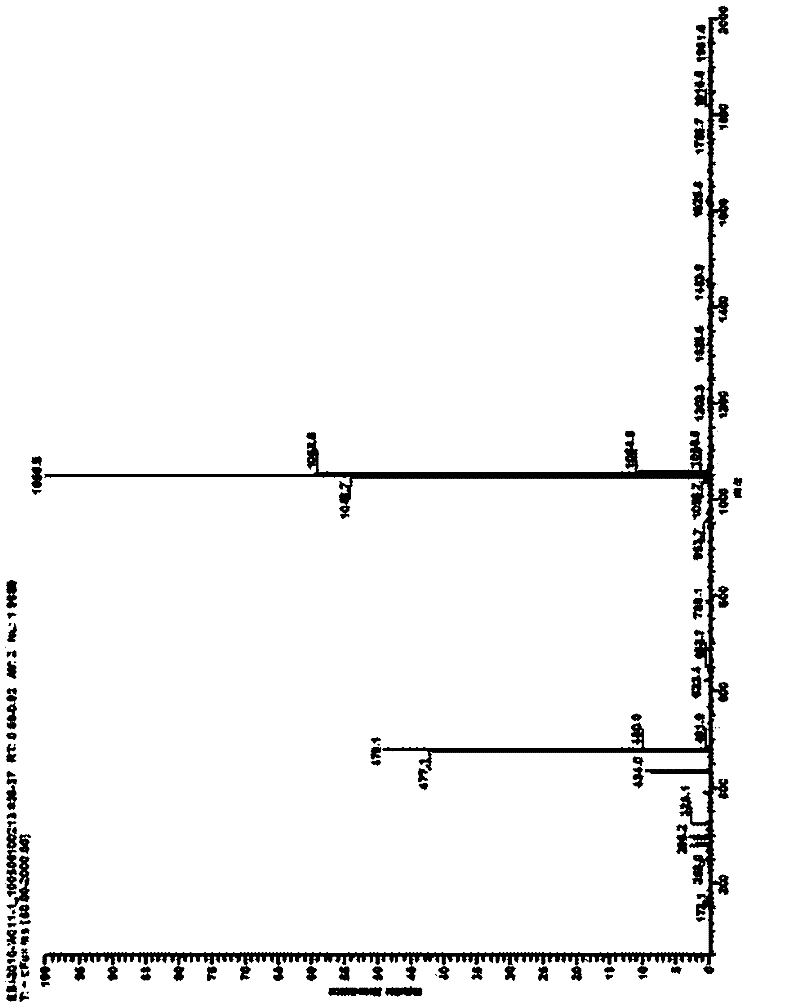
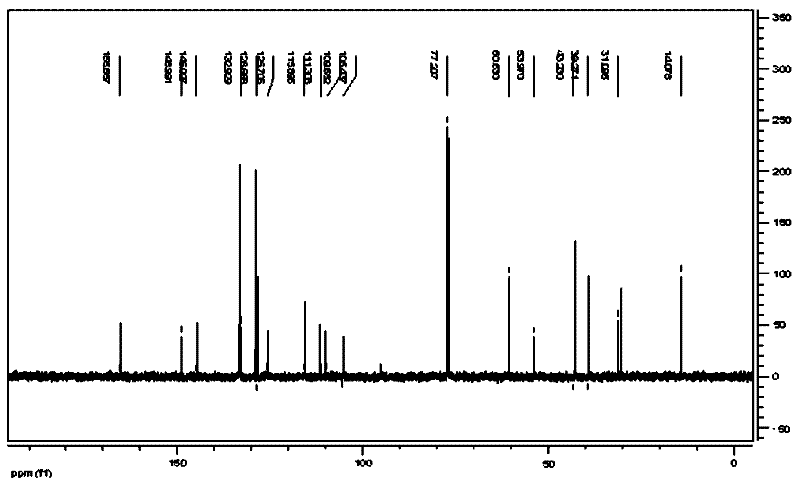
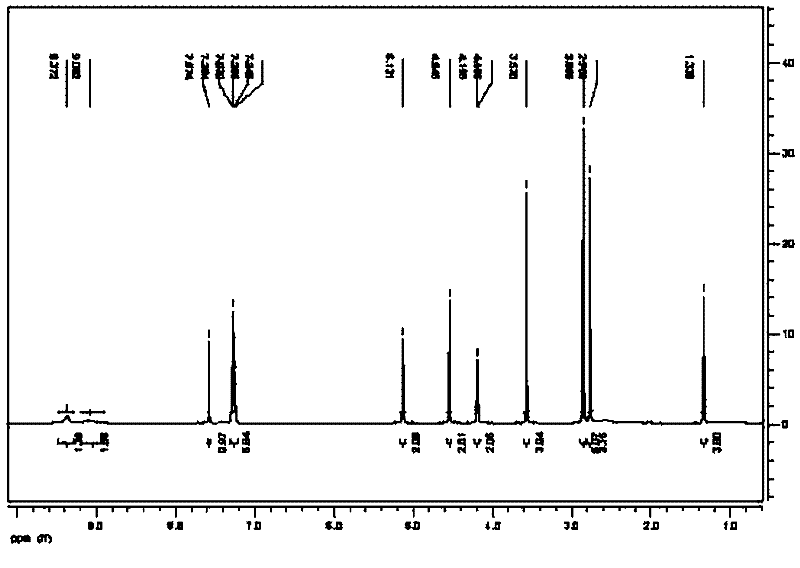
Image
Examples
Embodiment 20
[0158] A kind of synthetic method of Arbidol mesylate, its steps are as follows:
[0159] 1) Preparation of 3-methylamino-butenoic acid ethyl ester
[0160] Put 150.0 g of ethyl acetoacetate into the reaction bottle, add 160 mL of 40 wt % methylamine aqueous solution dropwise under stirring at 25 ° C, after the drop is complete, continue to stir and react at this temperature for 3 hours, let stand to separate layers, and wash the lower organic layer with water , and dried to obtain intermediate (B) 157.6g, yield: 95.5%.
[0161] 2) Preparation of 1,2-dimethyl-5-oxindole-3-carboxyethyl ester
[0162] Dissolve 124.0g of p-benzoquinone in 1200mL of acetone in a reaction flask, stir evenly, heat to 30°C, add 150.0g of intermediate (B) dropwise under stirring, and continue to react for 2 hours. 2 / 3 of the solvent was cooled to 0-5° C., filtered to obtain a solid, and dried to obtain 90.1 g of intermediate (C), yield: 36.9%.
[0163]3) Preparation of 6-bromo-2-bromomethyl-5-oxind...
Embodiment 21
[0173] A kind of synthetic method of Arbidol mesylate, its steps are as follows:
[0174] 1) Preparation of 3-methylamino-butenoic acid ethyl ester
[0175] Put 100 g of ethyl acetoacetate into the reaction bottle, add 120 mL of 40 wt % methylamine aqueous solution dropwise under stirring at 30 ° C, after the drop is completed, continue to stir and react at this temperature for 3 hours, let stand to separate layers, and wash the lower organic layer with water. Dry to obtain intermediate (B) 99.0 g, yield: 90.0%.
[0176] 2) Preparation of 1,2-dimethyl-5-oxindole-3-carboxyethyl ester
[0177] Dissolve 74.7g of p-benzoquinone in 700mL of acetone in a reaction flask, stir evenly, heat to 40°C, add 90.0g of intermediate (B) dropwise under stirring, and continue to react for 2 hours. 2 / 3 of the solvent was cooled to 0-5° C., filtered to obtain a solid, and dried to obtain 52.3 g of intermediate (C), yield: 37.7%.
[0178] 3) Preparation of 6-bromo-2-bromomethyl-5-oxindole-3-carb...
Embodiment 22
[0187] A kind of synthetic method of Arbidol mesylate, its steps are as follows:
[0188] 1) Preparation of 3-methylamino-butenoic acid ethyl ester
[0189] Put 153.2 g of ethyl acetoacetate into the reaction bottle, add 180 mL of 40 wt % methylamine aqueous solution dropwise under stirring at 35 ° C, after the drop is complete, continue to stir and react at this temperature for 3 hours, let stand to separate layers, and wash the lower organic layer with water , and dried to obtain intermediate (B) 161.9g, yield: 96.14%.
[0190] 2) Preparation of 1,2-dimethyl-5-oxindole-3-carboxyethyl ester
[0191] Dissolve 132.9g of p-benzoquinone in 1300mL of acetone in a reaction flask, stir evenly, heat to 45°C, add 160.0g of intermediate (B) dropwise under stirring, and continue to react for 2 hours. 2 / 3 of the solvent. After cooling to 0-5°C, the solid was obtained by filtration and dried to obtain 91.2 g of intermediate (C), yield: 35.0%.
[0192] 3) Preparation of 6-bromo-2-bromo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com