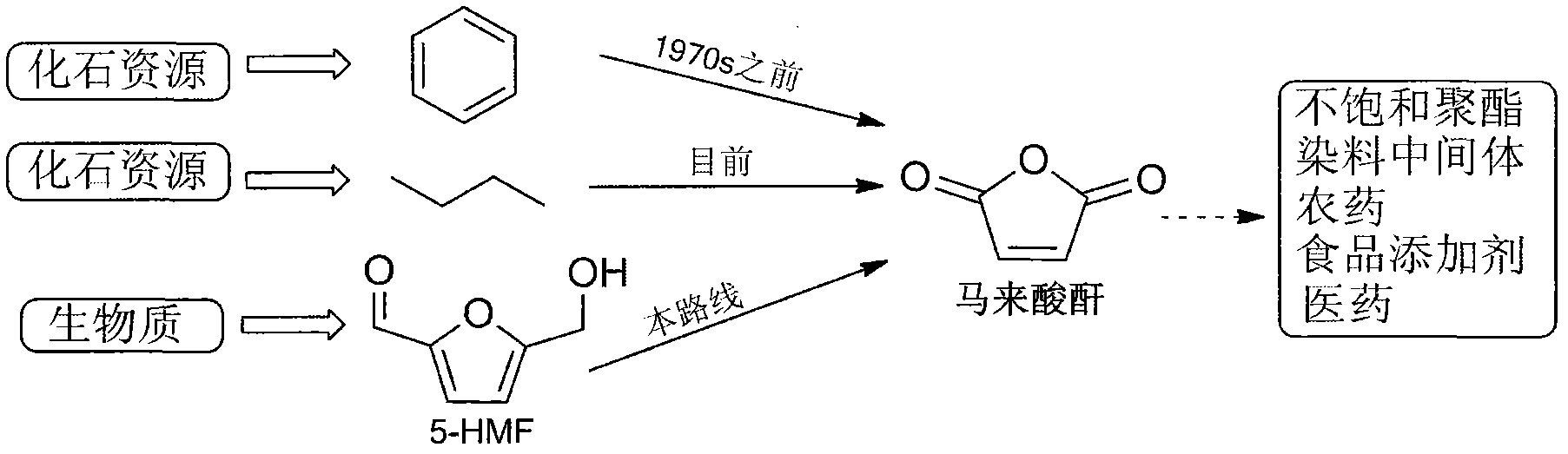
Method for preparing maleic anhydride by catalytic oxidation of 5-hydroxymethylfurfural
A technology of hydroxymethylfurfural and maleic anhydride, applied in the direction of organic chemistry, etc., to achieve the effects of safety and easy operation, mild reaction conditions and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
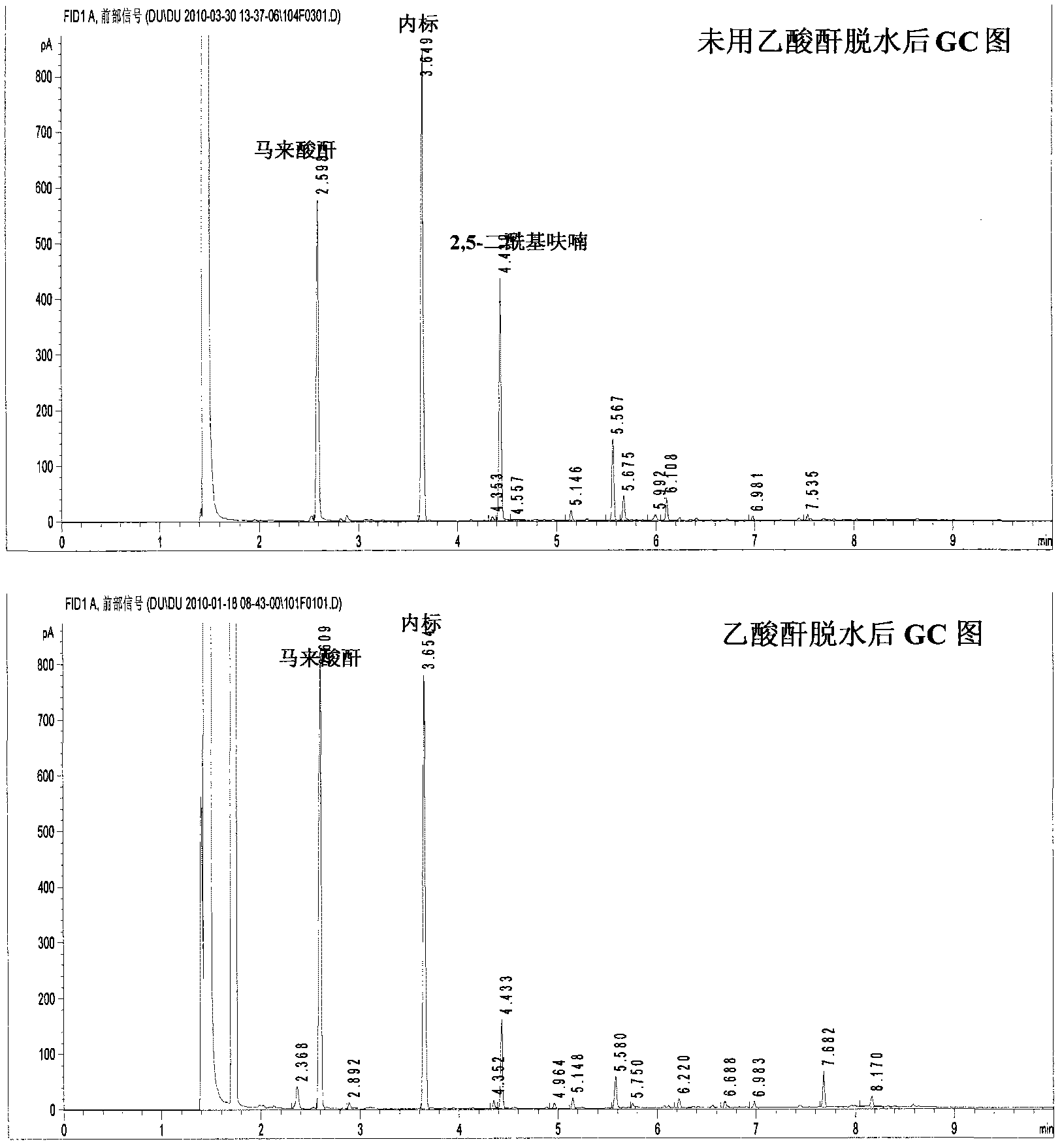
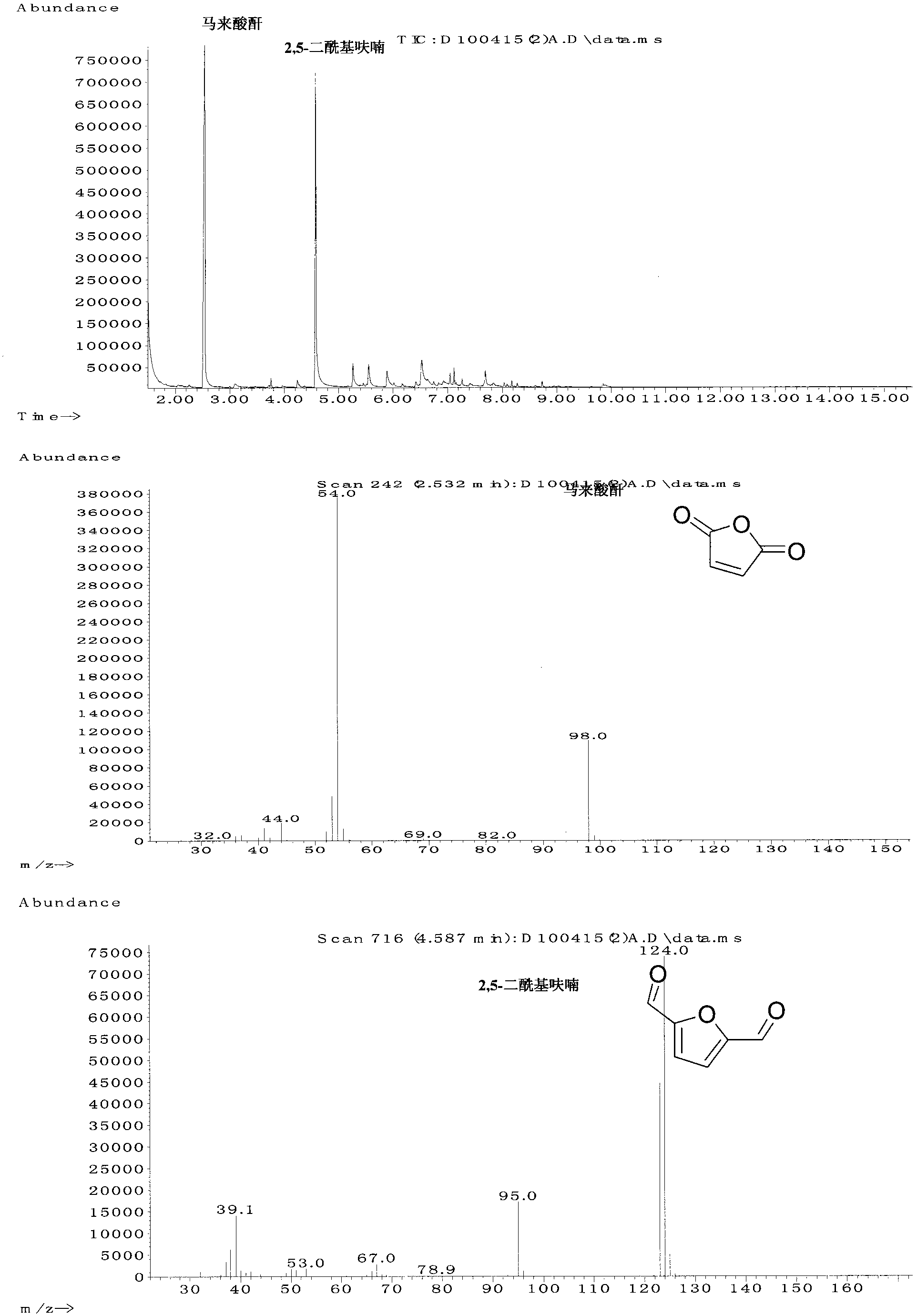
[0016] Example 1: Add 0.3150g 5-hydroxymethylfurfural and 5mol% vanadyl acetylacetonate to a 50mL reaction kettle, add 5mL acetonitrile, close the kettle, fill with oxygen at a pressure of 1.0MPa, and heat to 90°C under stirring. And keep it for 4h. Then cool to room temperature and carefully reduce pressure to normal pressure. Transfer all the products to a volumetric flask, add the internal standard 2,3,5,6-tetramethylbenzene and use acetone to make the volume, and then take a sample and use the gas chromatography (GC) internal standard quantitative method to obtain the maleic anhydride in the product. content. According to the formula, the yield of maleic anhydride = (the amount of maleic anhydride) / (the amount of the material 5-hydroxymethyl furfural), the yield of maleic anhydride is 43.4% ( figure 2 on). If 5mL acetic anhydride is added, the product is heated to reflux for 2h, that is, the maleic acid in the product is first converted into maleic anhydride, and the chr...
Embodiment 2
[0017] Example 2: Add 0.3150g 5-hydroxymethylfurfural and 5mol% mytol vanadyl into a 50mL reaction kettle, add 5mL acetonitrile, close the kettle, fill with oxygen at a pressure of 1.0MPa, and heat up to 90°C under stirring , And keep it for 4h. Then cool to room temperature and carefully reduce pressure to normal pressure. The product was analyzed according to the method in Example 1, and the total yield of maleic anhydride and maleic acid was 43.3%.
Embodiment 3
[0018] Example 3: 0.3150g 5-hydroxymethylfurfural and 5mol% vanadyl picolinate were added to a 50mL reaction kettle, 5mL acetic acid was added, the kettle was closed, the oxygen pressure was 0.4MPa, and the temperature was raised to 60°C while stirring , And keep it for 8h. Then cool to room temperature and carefully reduce pressure to normal pressure. The product was analyzed according to the method in Example 1, and the total yield of maleic anhydride and maleic acid was 29.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com