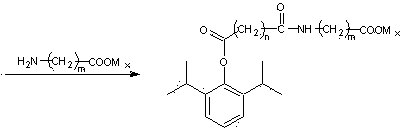
Propofol ester derivative containing amino carboxylic acid amide structure, its preparation method and its purpose
A technology of aminocarboxylic acid amide and propofol, which is applied to the preparation of carboxylic acid amide, the preparation of organic compounds, and medical preparations containing active ingredients, etc. It can solve the problems of poor water solubility, achieve stable aqueous solution, and have cheap and easy-to-obtain raw materials , reducing the effect of the first-pass effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
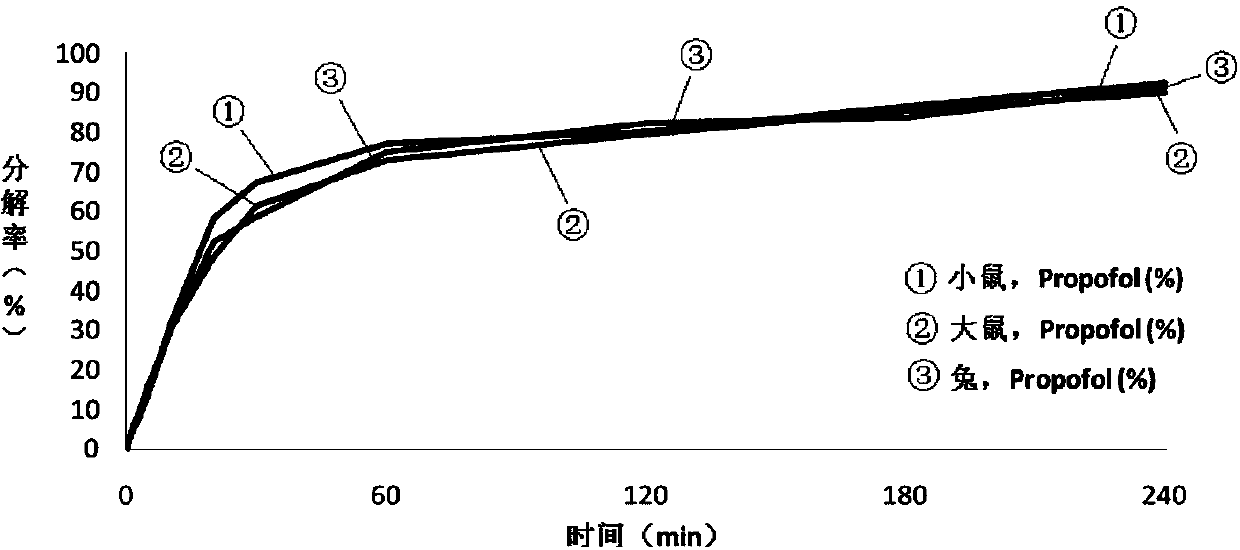
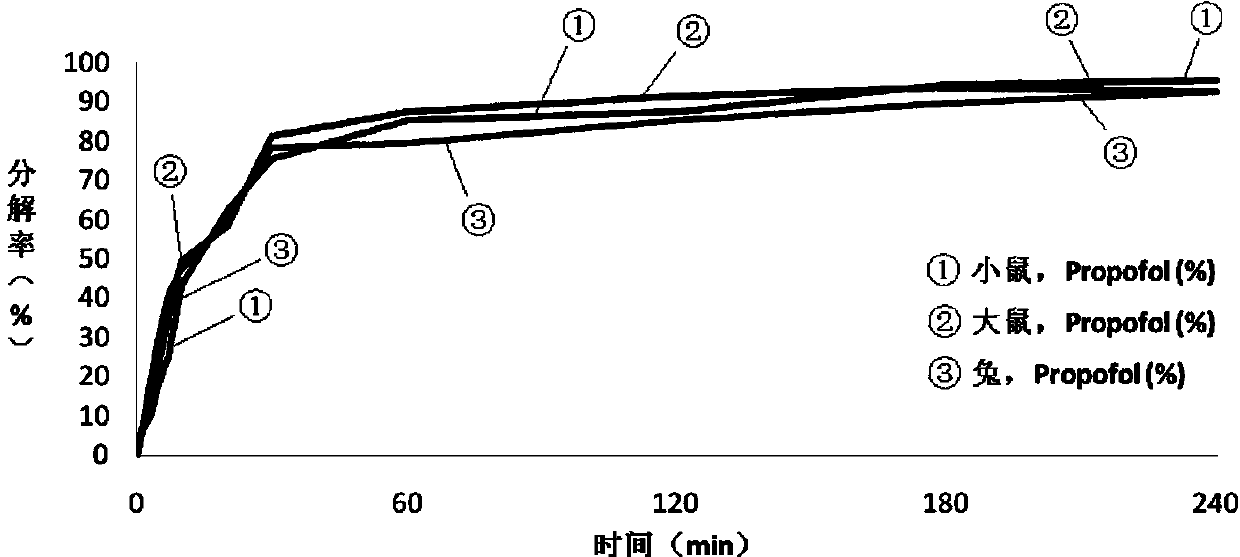
Image
Examples
Embodiment 1
[0039] Solk the 20 G propyphenol (type Ⅱ) in 50 mL triamine, add 14 G amber acidic anhydride and 0.02 G ditrobyl pyridine, stir at room temperature for 16 h, remove the reactor decompression distillation and remove excessive triamines with excessive amounts of totoramine, Remnant into 100 ml of water, adjust the pH value of 6 n n N nn nnid hydrochloric acid to 1, and precipitate a large amount of white precipitation.Acetate ethyl acetic crystals obtained a needle-shaped crystal 23.5 g, the reaction production rate was 75.4%, and MP: 103-104 ° C.
Embodiment 2
[0041] Solk the 20 G propyphenols in 100 ml of dichloromethane, add 13.3 g -b -t -t -t -methalic acid and 0.02 g di metrophic pyridine, then add 23.2 G DCC to the room temperature for 6 h, and then filter out the white solid in the reaction solution., Wash the filtrate with 150 ml 6N hydrochloric acid once, separate the organic layer, reduce the stamina and steam the solvent, obtain the light yellow solid of the propyel picoline monoclodine (Ⅲ).The white needle-shaped crystal is 26.6 g, the yield is 85%, MP: 102-103 ° C.
Embodiment 3
[0043] Called 1 g of proper phenols (Ⅲ), a propylene phenolic acid single ester (Ⅲ) in 25 mL mono bottle, add 10 ml of dry dichloroblyne, and quickly add 2 G new steamed strawlllchriumThe atmosphere reacts at the room temperature by 2 h. After the HCL is released, it is steamed to dry the solvent and low volatile substances on the rotary evaporator, that is, the propylene phenol ambercotate is single -chloride, and it is directly used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com