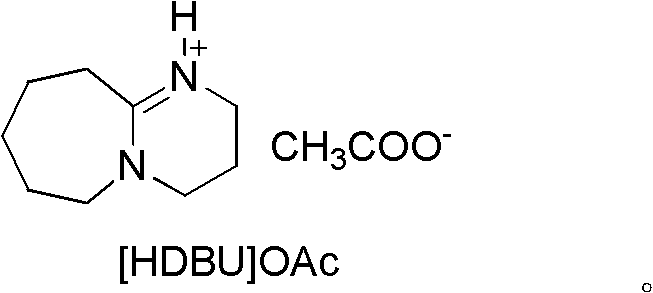Method for acetylating catalytic hydroxyl
A technology for catalyzing hydroxyacetylation and acetylation, applied in chemical instruments and methods, preparation of esterified saccharides, organic compounds, etc., can solve the problems of expensive reagents, harsh reaction conditions, long reaction time, etc., and achieve a green and friendly reaction. , the effect of short reaction time and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
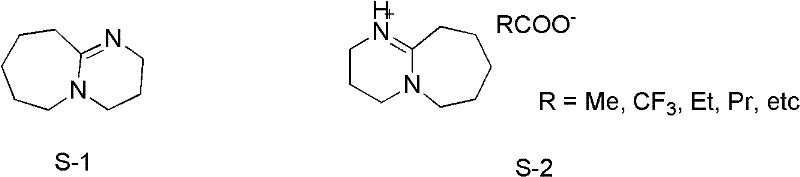
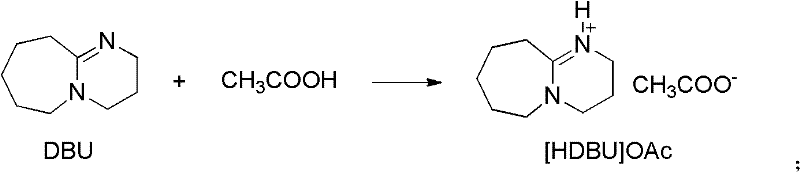
[0029] Embodiment 1, preparation of ionic liquid [HDBU]OAc, carry out the following steps successively:
[0030] Add 7.6g (50mmol) DBU into a 50mL three-neck flask, add an ice-water bath, and start magnetic stirring. Measure 3.0 g (50 mmol) of glacial acetic acid, transfer it to a constant-pressure dropping funnel, and slowly drop it into a flask while keeping the reaction temperature below 5°C (controlled at 0-5°C). After the addition of glacial acetic acid was completed, the ice-water bath was removed, and the reaction was stirred at room temperature for 24 h. The obtained oily reactant was vacuum-dried at 60° C. for 24 h to obtain a light yellow viscous liquid, namely Lewis base ionic liquid [HDBU]OAc, which was stored in a desiccator.
[0031] Its structural formula is as follows:
[0032]
Embodiment 2
[0033] Embodiment 2, a method for hydroxyacetylation catalyzed by a Lewis alkali ionic liquid (that is, a method for catalyzing hydroxyacetylation), using Lewis alkali ionic liquid [HDBU]OAc as a catalyst, the following steps are carried out successively:
[0034] 4-Nitrophenol (1.39g, 10mmol) and freshly distilled acetic anhydride (1.03g, 10.1mmol) were added to a 25mL flask equipped with a spherical condenser, followed by the catalyst [HDBU]OAc (0.42g, 2mmol, i.e. 20% molar ratio), turn on magnetic stirring, and slowly heat up to 50°C. The progress of the reaction was monitored by TLC (developing solvent: EtOAc / hexanes=1:4, V / V) and GC. After 45min, the conversion of 4-nitrophenol was completed. Add 20mL of diethyl ether to dilute the reaction solution, and filter to separate the upper product. 3 After washing the solution with 10 mL of water, add 0.5 g of anhydrous Na 2 SO 4 Drying, rotary evaporation under reduced pressure (0.02MPa) to remove diethyl ether yielded 1.72g...
Embodiment 3
[0035] Embodiment 3, a kind of hydroxyacetylation method catalyzed by Lewis alkali ionic liquid, take Lewis alkali ionic liquid [HDBU]OAc as catalyst, carry out the following steps successively:
[0036] Phenol (0.94 g, 10 mmol) and freshly distilled acetic anhydride (1.03 g, 10.1 mmol) were added to a 25 mL flask equipped with a spherical condenser, followed by the catalyst [HDBU]OAc (0.42 g, i.e. 20% molar ratio) , turn on the magnetic stirring, and slowly raise the temperature to 50°C. The progress of the reaction was monitored by TLC (developing solvent: EtOAc / hexanes=1:4, V / V) and GC. After 45min, the conversion of phenol was completed, and 20mL of diethyl ether was added to dilute the reaction solution, filtered, and the upper product was separated, and the obtained organic phase (that is, the upper layer extract containing the product) was successively washed with 10mL saturated NaHCO 3 After washing the solution with 10 mL of water, add 0.5 g of anhydrous Na 2 SO 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com