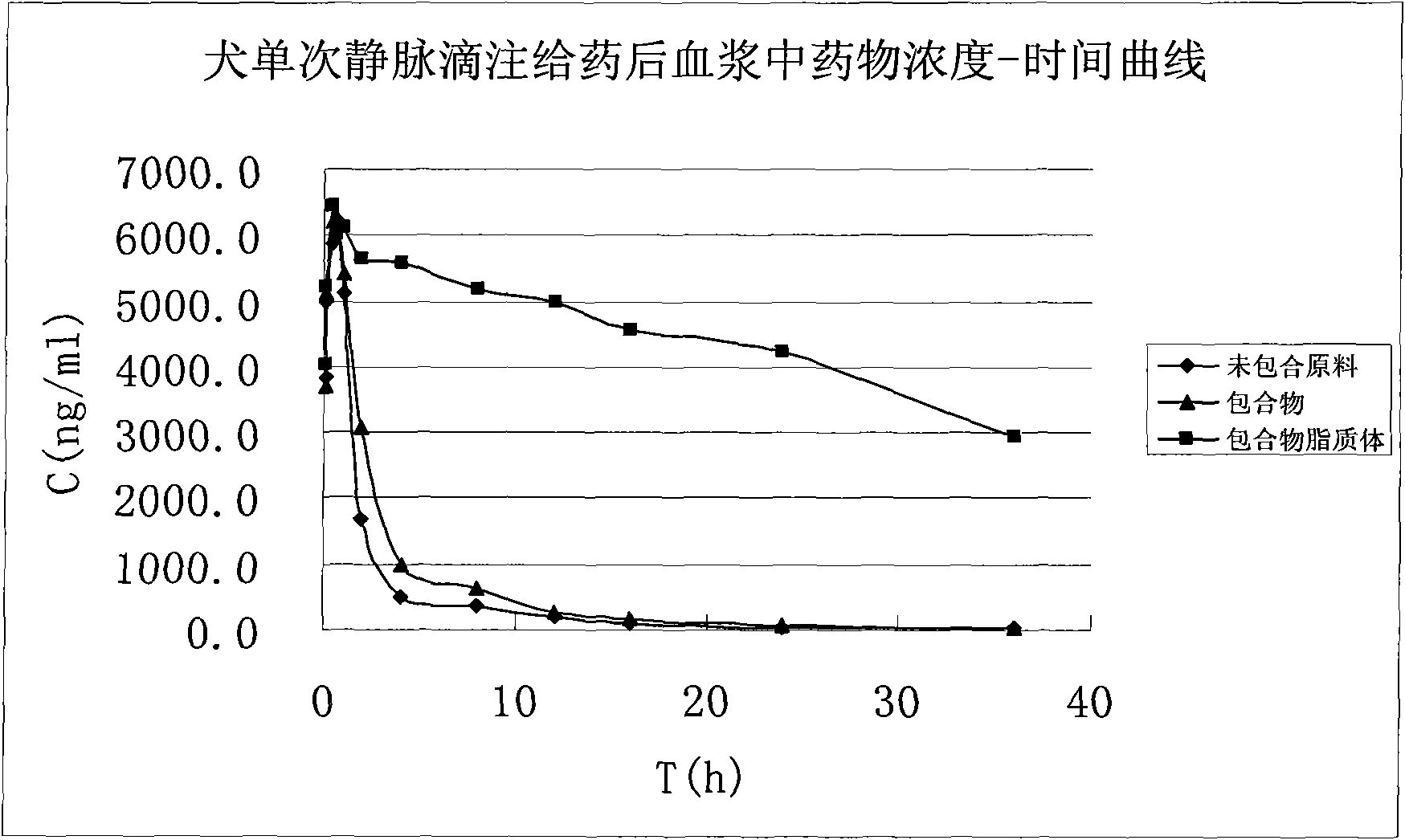
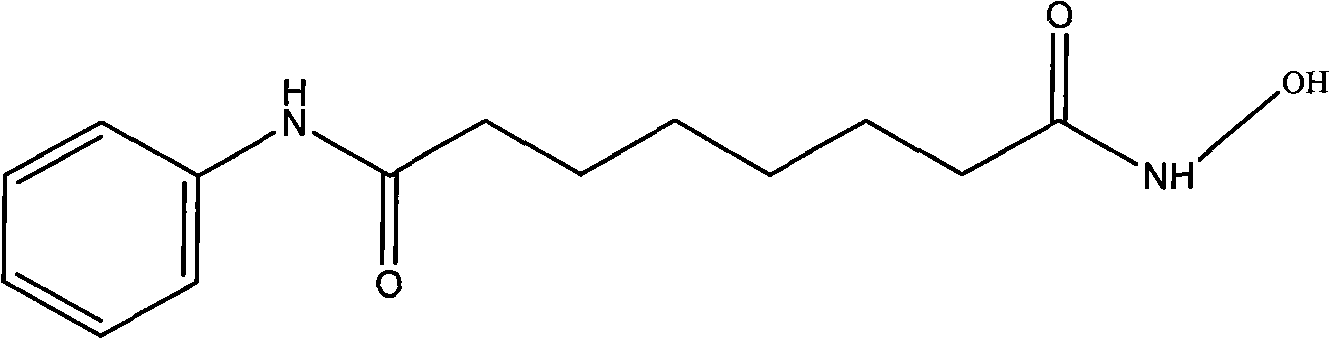
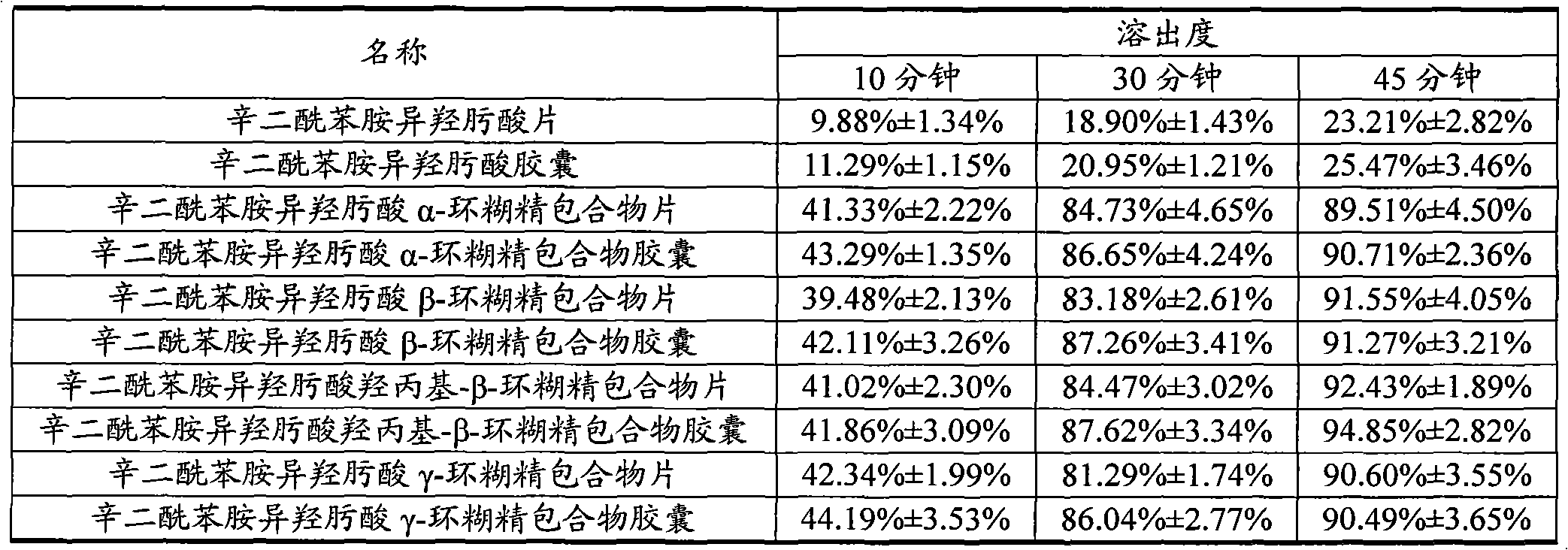
Octanedioyl benzohydroxamic acid cyclodextrin clathrate liposome
A technology for inclusion complex of suberoylanilide hydroxime and cyclodextrin, which is applied in the application field of pharmaceutical preparations, can solve the problems of slow onset, poor water solubility of suberoylanilide hydroxamic acid and the like, and achieves delayed release , Improve the drug-to-lipid ratio, increase the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The formula that present embodiment adopts is as follows:
[0040] Suberoylanilide hydroxamic acid 10mg
[0041] Hydroxypropyl-β-cyclodextrin 150mg
[0042] Lecithin 200mg
[0043] Cholesterol 60mg
[0044] Weigh the prescribed amount of suberoylanilide hydroxamic acid and hydroxypropyl-β-cyclodextrin, first dissolve the hydroxypropyl-β-cyclodextrin in an appropriate amount of water to form a saturated solution, control the temperature at about 60°C, Stir constantly. Suberoylanilide hydroxamic acid is ground with a small amount of alcohol, slowly added dropwise to the above-mentioned cyclodextrin saturated solution with constant stirring, filtered, and the filtrate is vacuum-dried. Dissolve the prepared suberoylanilide hydroxamic acid clathrate together with the prescribed amount of lecithin and cholesterol in an appropriate amount of chloroform, remove the solvent by rotary evaporation under reduced pressure at 50°C, and continue rotary evaporation under reduced pr...
Embodiment 2
[0046] The formula that present embodiment adopts is as follows:
[0047] Suberoylanilide hydroxamic acid 20mg
[0048] Dimethyl-beta-cyclodextrin 350mg
[0049] Lecithin 280mg
[0050] Weigh the prescribed amount of suberoylanilide hydroxamic acid and dimethyl-β-cyclodextrin, dissolve suberoylanilide hydroxamic acid in an appropriate amount of methanol, blow dry with nitrogen stream to remove the solvent, and dissolve the cyclodextrin Saturated aqueous solution was added to the above dried drug film, and the dispersion of the clathrate was centrifuged at 70,000 g for 60 min, the unclad precipitate in the lower layer was filtered off, and the filtrate was dried. Wet the prepared suberoylanilide hydroxamic acid inclusion compound with a small amount of water, vortex mix with phospholipids at 60°C for 2 minutes, add appropriate amount of water to dilute, freeze-dry, add a small amount of water to the freeze-dried solid, hydrate at high speed Centrifuge (12000rpm × 10min), tak...
Embodiment 3
[0052] The formula that present embodiment adopts is as follows:
[0053] Suberoylanilide hydroxamic acid 12mg
[0054] Hydroxypropyl-β-cyclodextrin 180mg
[0055]Soy Lecithin 240mg
[0056] Cholesterol 70mg
[0057] Vitamin C 5mg
[0058] Mannitol 22mg
[0059] pH adjusted to 6.5-8.0
[0060] Water for injection to the full amount of 1000ml
[0061] Weigh the prescribed amount of suberoylanilide hydroxamic acid and hydroxypropyl-β-cyclodextrin, first dissolve the hydroxypropyl-β-cyclodextrin in an appropriate amount of water for injection to form a saturated solution, and control the temperature at about 60 °C, stirring continuously. Suberoylanilide hydroxamic acid is ground with a small amount of alcohol, slowly added dropwise to the above-mentioned cyclodextrin saturated solution with constant stirring, filtered, and the filtrate is vacuum-dried. Dissolve the prepared suberoylanilide hydroxamic acid clathrate together with the prescribed amount of lecithin and chole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com