Method for synthesizing azoxystrobin intermediate
A synthesis method and intermediate technology, applied in the field of synthesis of azoxystrobin intermediates, can solve the problems of difficult reaction, high reaction temperature, and many side reactions, and achieve mild reaction conditions, high reaction yield, and simple synthesis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
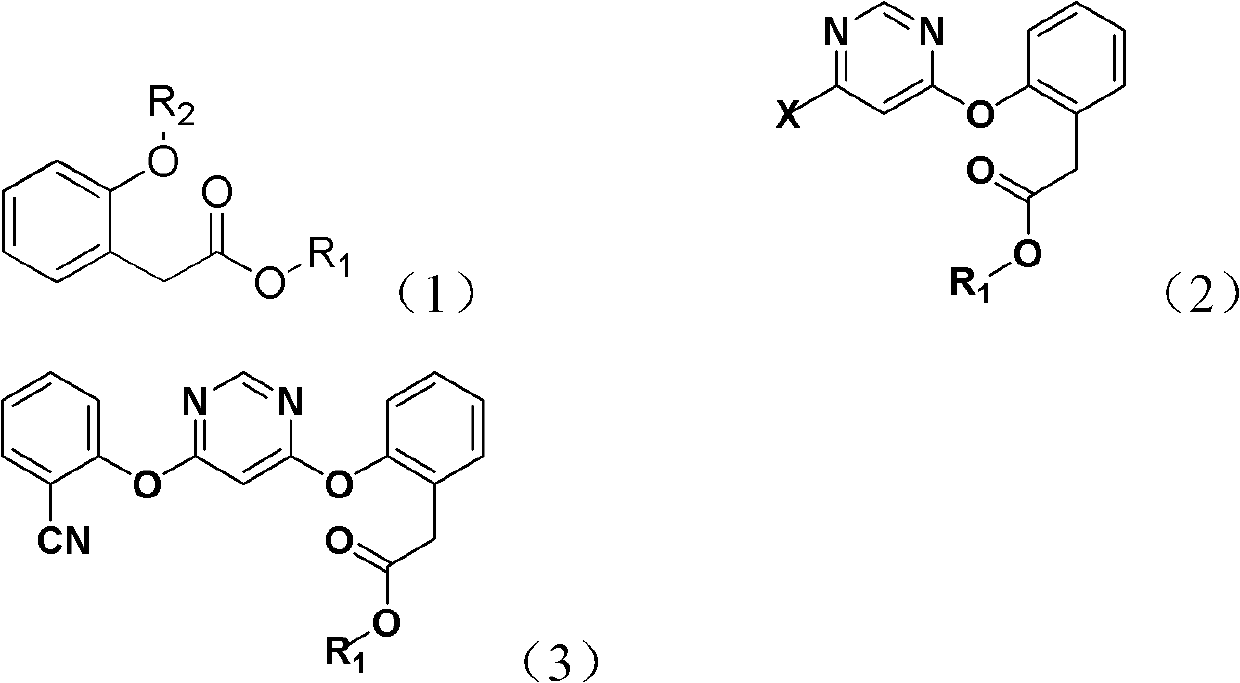
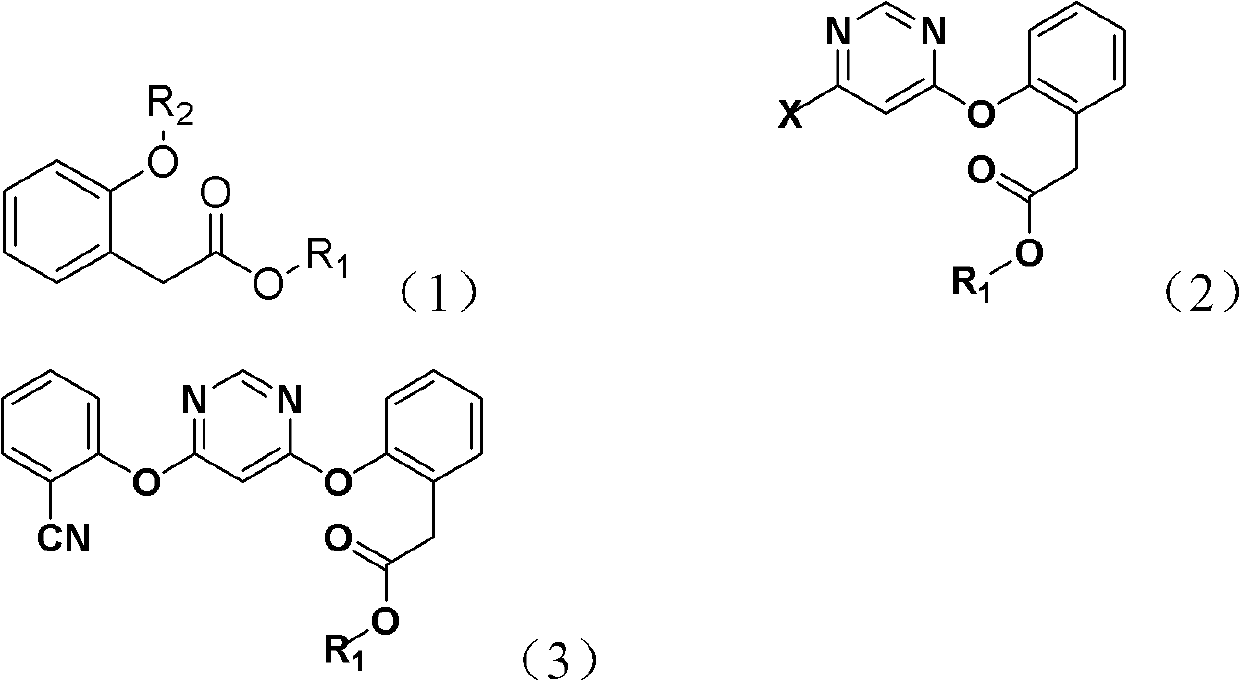
[0021] The present invention provides a method for synthesizing an azoxystrobin intermediate, the azoxystrobin intermediate having a structure represented by formula (3), wherein the method includes the following steps:
[0022] 1) Under basic conditions, the 4,6-dihalopyrimidine and the compound represented by formula (1) are subjected to the first contact reaction to obtain a product containing the compound represented by formula (2);
[0023] 2) Under alkaline conditions, in the presence of a catalyst, the compound of formula (2) is subjected to a second contact reaction with 2-hydroxybenzonitrile to obtain azoxystrobin containing the structure of formula (3). Body product
[0024]
[0025] In formulas (1), (2) and (3), R 1 Is methyl, ethyl or propyl; R 2 Is hydrogen, potassium or sodium; X is halogen; the catalyst is 1,4-diazabicyclo[2,2,2]octane and / or 2-methyl-1,4-diazabicyclo [2,2,2]octane.
[0026] The present invention provides a method for synthesizing an azoxystrobin inter...
Embodiment 1
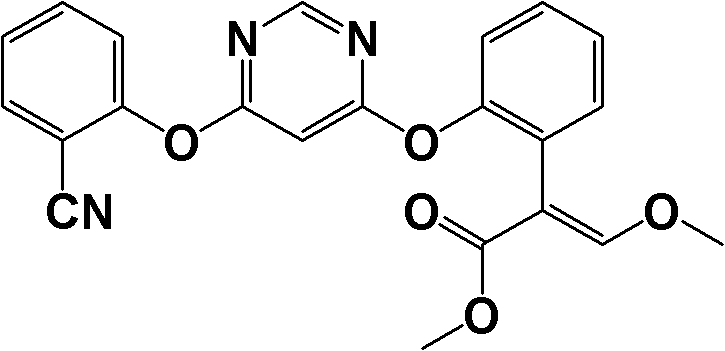
[0055] Under the protection of nitrogen, 200ml of dry N,N-dimethylformamide (water content less than 0.5% by weight, the same below), methyl 2-hydroxyphenylacetate (33.2g, 0.2mol), 4,6-Dichloropyrimidine (29.8g, 0.2mol) and anhydrous potassium carbonate (27.6g, 0.20mol). Turn on the stirring, heat to 50°C, stir and react for 2 hours. Subsequently, 2-hydroxybenzonitrile (23.8g, 0.2mol), anhydrous potassium carbonate (22.1g, 0.16mol) and DABCO (0.114g, 0.001mol) were added, the temperature was raised to 80°C, and the reaction was stirred for 3 hours. Then, after concentration under reduced pressure to recover N,N-dimethylformamide, 150ml of dichloromethane and 100ml of water were added to the residue, stirred to dissolve the solid, after standing to separate the layers, the organic phase was separated and concentrated to obtain a solid , Add 150ml methanol to the solid and recrystallize to obtain 68.7g of yellow solid, namely methyl 2-(2-(6-(2-cyanophenoxy)pyrimidine-4-oxy)pheny...
Embodiment 2
[0061] It was carried out according to the method of Example 1, except that the amount of DABCO used was 0.045g (0.0004mol). 67.8 g of methyl 2-(2-(6-(2-cyanophenoxy)pyrimidine-4-oxy)phenyl)acetate was obtained as a yellow solid, and its melting point was determined to be 95-96°C; 1 H NMR(500MHz, CDCl 3 ): δ 3.61 (s, 3H), 3.62 (s, 2H), 6.54 (d, 1H), 7.16 (q, 1H), 7.28 (m, 1H), 7.33 (m, 1H), 7.32-7.41 ( m, 3H), 7.67 (m, 1H), 7.23 (q, 1H), 8.39 (d, 1H). In addition, the total yield of the reaction and the purity of the yellow solid are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com