Preparation method of lamellar stannic sulfide/silicon oxide nuclear shell nanorod for lithium battery
A silicon dioxide and tin disulfide technology, applied in the field of materials science, can solve the problems of difficult synthesis of layered tin disulfide nanorods, and achieve the effects of facilitating commercial application, buffering volume expansion, and improving cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Add 0.05 gram of tin nanorods to 120 milliliters of ethanol solution, ultrasonic for 5 minutes, then add 20 milliliters of water, 20 milliliters of ammonia and 30 microliters of tetraethyl orthosilicate solution, and react at room temperature for 60 minutes to obtain tin / Silica core-shell nanorods; NH in ammonia used 3 The mass percentage content is 25 to 28%, and the SiO in the tetraethyl orthosilicate solution used 2 The mass percentage content is higher than 28.0%;
[0025] (2) Place 0.5 gram of tin / silicon dioxide core-shell nanorods obtained in step (1) in a tube furnace for vulcanization reaction, the sulfur source used is 1 gram of hydrogen sulfide gas, the reaction temperature is 400 ° C, and the reaction time is After 360 minutes, layered tin disulfide / silica core-shell nanorods were obtained.
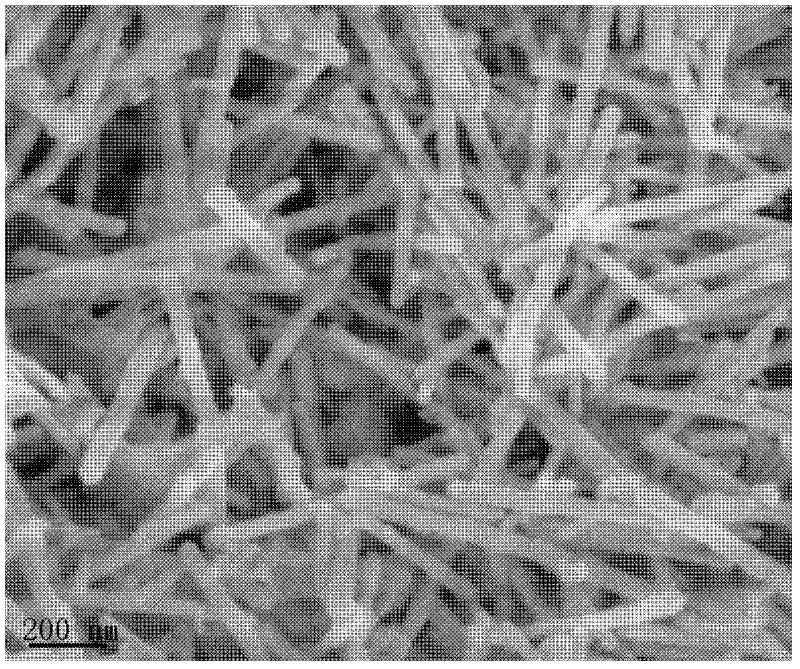
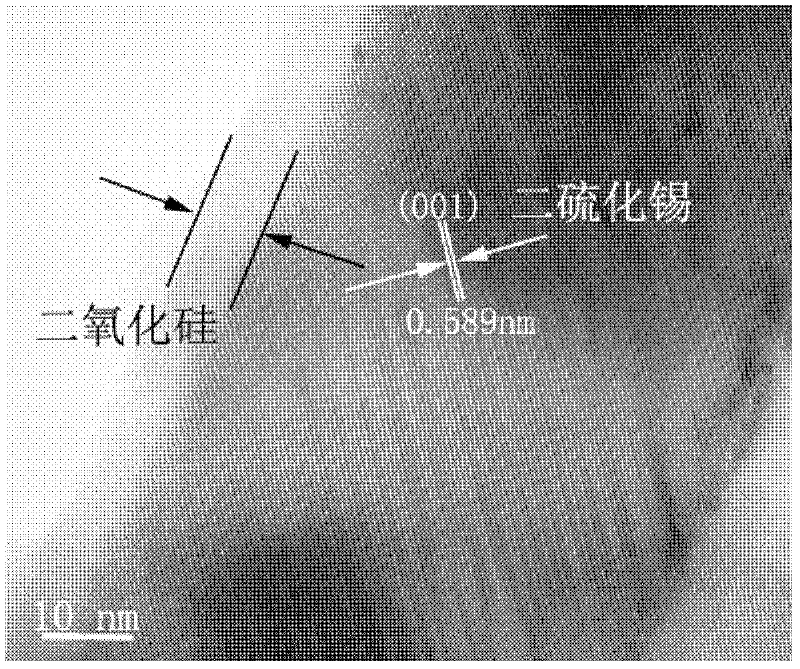
[0026] Figure 1 ~ Figure 4 These are scanning electron micrographs, transmission electron micrographs, high-resolution transmission electron micrographs and X-...
Embodiment 2
[0028] (1) Add 0.005 gram of tin nanorods to 20 milliliters of ethanol solution, sonicate for 1 minute, then add 5 milliliters of water, 5 milliliters of ammonia and 5 microliters of ethyl orthosilicate solution successively, and react at room temperature for 30 minutes to obtain tin / Silica core-shell nanorods; NH in ammonia used 3 The mass percentage content is 25 to 28%, and the SiO in the tetraethyl orthosilicate solution used 2 The mass percentage content is higher than 28.0%;
[0029] (2) Place 0.05 gram of tin / silicon dioxide core-shell nanorods obtained in step (1) in a tube furnace for vulcanization reaction. The sulfur source used is 5 grams of sulfur powder, the reaction temperature is 450 ° C, and the reaction time is 30 Minutes, layered tin disulfide / silica core-shell nanorods were obtained. The result is similar to Example 1.
Embodiment 3
[0031] (1) 0.5 grams of tin nanorods are added to 1 liter of ethanol solution, ultrasonicated for 30 minutes, followed by adding 200 milliliters of water, 200 milliliters of ammonia and 200 microliters of ethyl orthosilicate solution, and reacting at room temperature for 720 minutes to obtain tin / Silica core-shell nanorods; NH in ammonia used 3 The mass percentage content is 25 to 28%, and the SiO in the tetraethyl orthosilicate solution used 2 The mass percentage content is higher than 28.0%;
[0032] (2) 0.05 grams of tin / silica core-shell nanorods obtained in step (1) are placed in a tube furnace for vulcanization reaction, the sulfur source used is 0.5 grams of hydrogen sulfide, the reaction temperature is 500 ° C, and the reaction time is 720 Minutes, layered tin disulfide / silica core-shell nanorods were obtained. The result is similar to Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com