Method for preparing nitrogen-doped carbon nanotube fuel cell catalyst
A nitrogen-doped carbon, fuel cell technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., to achieve the effects of simple and easy method, avoidance of corrosion, and high-efficiency oxygen reduction catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
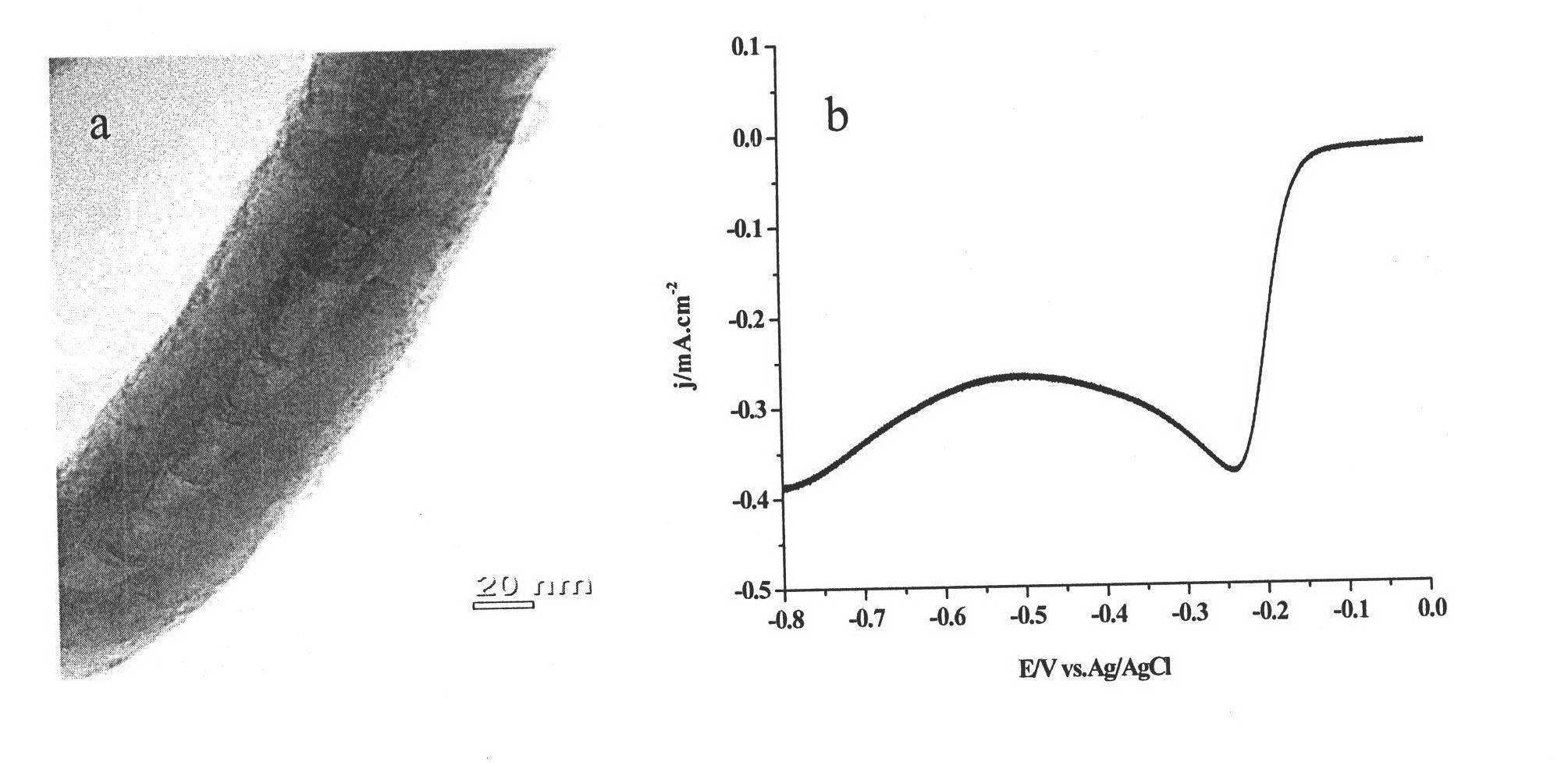
Embodiment 1
[0029] (1) Preparation of nitrogen-doped carbon nanotube catalyst
[0030] Weigh melamine and ferrocene according to the mass ratio of melamine:ferrocene as 1:2, grind and mix the melamine and ferrocene evenly and place them in the low temperature zone of the tube furnace, place the quartz sheet in the high temperature zone of the tube furnace zone, first pass nitrogen into the tube furnace for 0.5 hours to remove the air in the tube furnace. The nitrogen gas brings the sublimated precursor into the high-temperature zone, and the flow rate of the nitrogen gas is controlled to be 50 ml / min. After 40 minutes of reaction, nitrogen-doped carbon nanotubes are obtained.
[0031] (2) Purification of nitrogen-doped carbon nanotube catalysts
[0032] Oxidize the nitrogen-doped carbon nanotube catalyst prepared in step (1) at 200°C for 6 hours to remove the amorphous carbon therein, then heat and stir the above product with concentrated nitric acid to reflux for 5 hours, filter, wash, ...
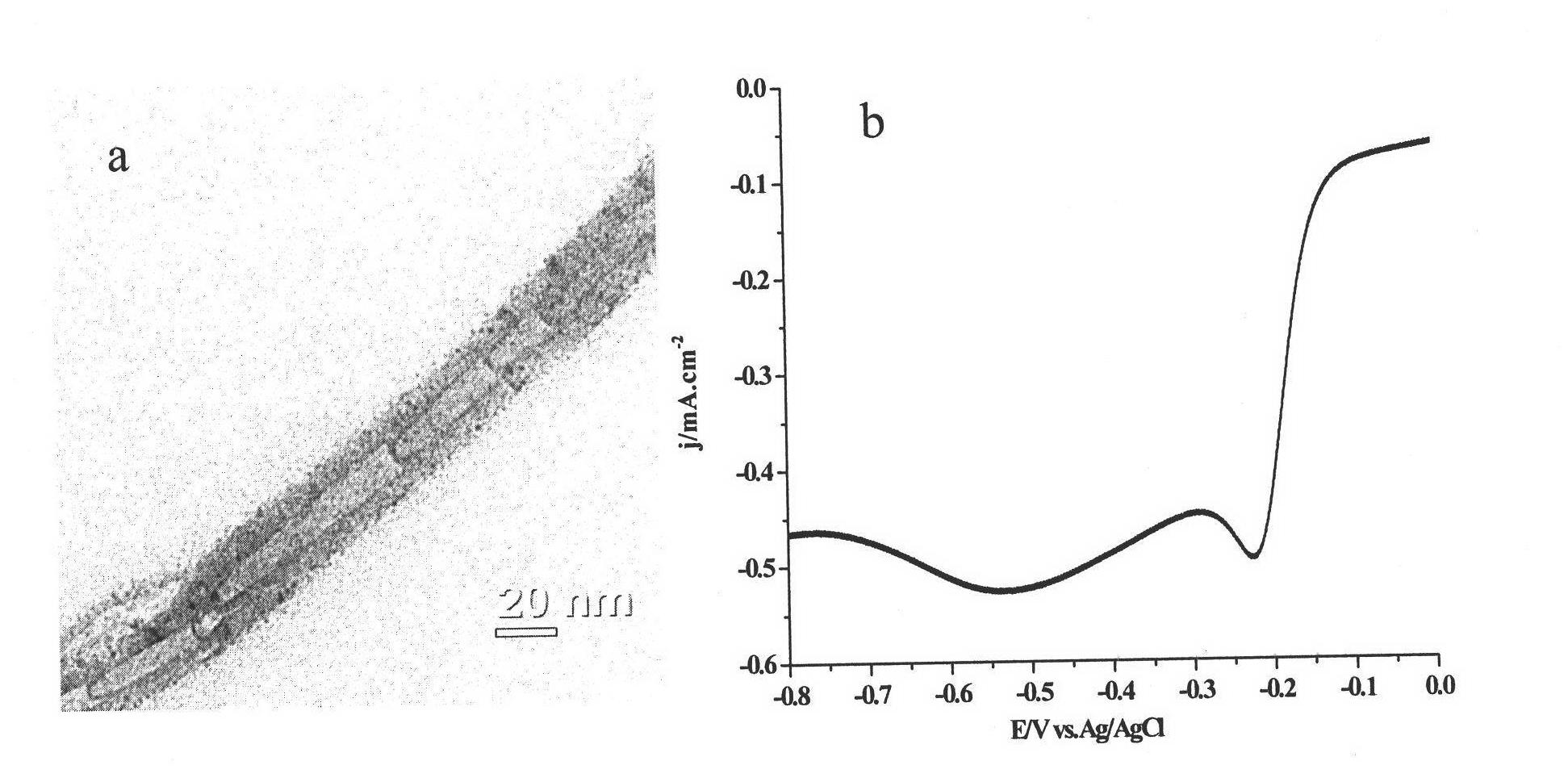
Embodiment 2
[0036] (1) Preparation of nitrogen-doped carbon nanotube catalyst
[0037]Weigh urea and ferrocene according to the mass ratio of urea:ferrocene as 1:4, grind and mix the urea and ferrocene evenly and place them in the low temperature zone of the tube furnace, place the quartz sheet in the high temperature zone of the tube furnace zone, first pass nitrogen into the tube furnace for 1 hour to remove the air in the tube furnace, after the ventilation is over, raise the temperature of the low temperature zone to 200°C, and the temperature of the high temperature zone to 600°C. The nitrogen gas brings the sublimated precursor into the high-temperature zone, and the flow rate of the nitrogen gas is controlled to be 100 ml / min. After 20 minutes of reaction, nitrogen-doped carbon nanotubes are obtained.
[0038] (2) Purification of nitrogen-doped carbon nanotube catalysts
[0039] Oxidize the nitrogen-doped carbon nanotube catalyst prepared in step (1) at 300°C for 3 hours to remove...
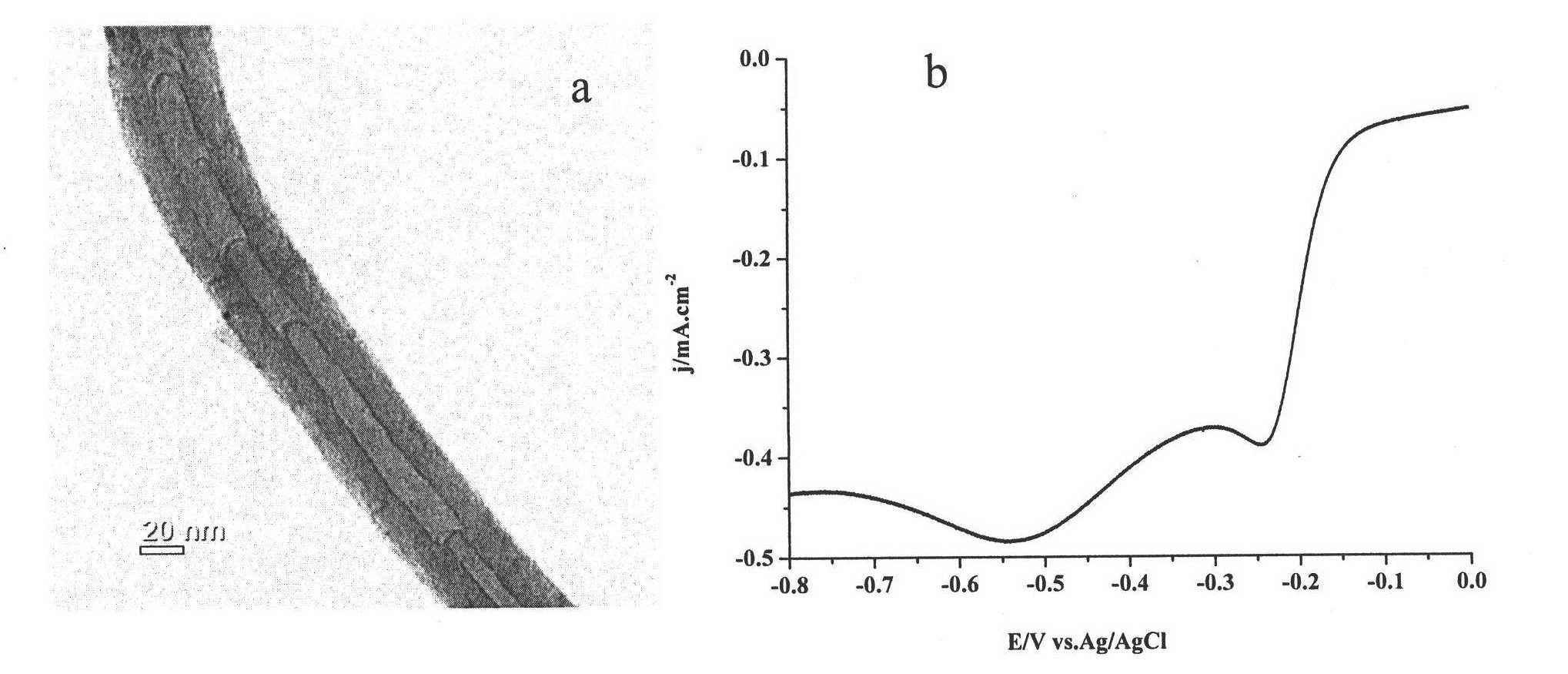
Embodiment 3
[0043] (1) Preparation of nitrogen-doped carbon nanotube catalyst
[0044] Weigh melamine and ferrocene according to the mass ratio of melamine:ferrocene as 1:1, grind and mix the melamine and ferrocene evenly and place them in the low temperature zone of the tube furnace, place the quartz sheet in the high temperature zone of the tube furnace zone, first pass 0.8 hours of argon into the tube furnace to remove the air in the tube furnace. Enter argon gas to bring the sublimated precursor into the high temperature zone, control the flow rate of argon gas to 150 ml / min, and obtain nitrogen-doped carbon nanotubes after 80 minutes of reaction.
[0045] (2) Purification of nitrogen-doped carbon nanotube catalysts
[0046] Oxidize the nitrogen-doped carbon nanotube catalyst prepared in step (1) at 400°C for 10 hours to remove the amorphous carbon, then heat and stir the above product with concentrated nitric acid to reflux for 5 hours, filter, wash and dry A purified nitrogen-dope...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com