Preparation method of 9,9-bis(4-aminophenyl)fluorene
A technology of aminophenyl and fluorenone, which is applied in the field of preparation of 9,9-bis(4-aminophenyl)fluorene, can solve the problems of difficult operation, large amount of recrystallization solvent, cumbersome process route, etc., and achieve inhibition The formation of by-products, mild method conditions, and the effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0013] The preparation method of this example is as follows: under nitrogen protection, add 3.6 g of fluorenone (20 mmol), 7.2 g of aniline hydrochloride (55.6 mmol), and 0.5 g to a 100 mL two-necked bottle equipped with a water separator sodium bisulfite (4.8 mmol), 8.8 mL of aniline (96.4 mmol) and 6 mL of toluene. Slowly rise to 120°C under stirring, react for 30 minutes, separate out the green liquid produced in the water separator, maintain the temperature for 3 hours, separate out the water produced in the water separator, then slowly raise the temperature to 135°C, maintain this temperature for reaction After 1.5 hours, the reaction can be stopped when no more water is produced in the water separator.
[0014] Cool the reacted material to about 60°C, add 10wt% potassium hydroxide solution to the mixed solution, adjust the pH value to 9, stir for 15 minutes while it is hot, then carry out suction filtration, and wash the filter cake with 30mL of toluene Recrystallizatio...
Embodiment 2~ Embodiment 3)
[0016] The preparation method of each embodiment is basically the same as that of Example 1, and the differences are shown in Table 1.
[0017]
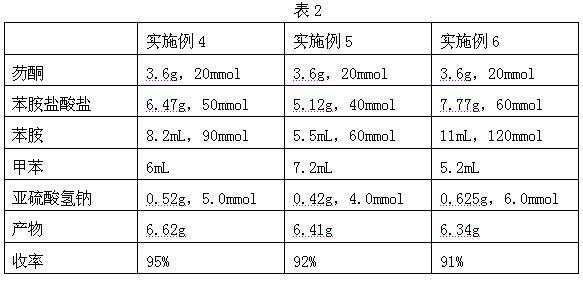
Embodiment 4~ Embodiment 6)
[0019] The preparation method of each embodiment is basically the same as that of Example 1, and the differences are shown in Table 2.
[0020]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com