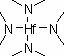
Synthetic method of tetra(dimethylamino)hafnium
A synthesis method, dimethylamine-based technology, applied in the field of synthesis of metal-organic complexes, can solve the problems of small lithium chloride salt particles, difficulty in separating lithium chloride salt, and difficulty in filtration, so as to simplify operations, reduce costs, Handle simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthesis of tetrakis (dimethylamino) hafnium
[0028] (1) Under an argon atmosphere, add 108 g of dimethylamine and 200 mL of n-hexane into a 2000 mL three-necked bottle, stir mechanically, and place the reaction bottle between -40 and -80 °C. Add 800 mL of 2.5 mol / L n-butyllithium solution dropwise into the reaction flask. After the dropwise addition, the reaction was stirred for 10 hours.
[0029] (2) Add 160 grams of hafnium tetrachloride in batches to the above reaction system, keeping the temperature of the reaction system not higher than 60°C. After adding the hafnium tetrachloride, let the reaction system react for 24-30 hours under the condition of mechanical stirring and inert gas protection.
[0030] (3) After the reaction is over, change the reaction device directly into a distillation device, remove the reaction solvent under an atmospheric pressure, wait for the solvent n-hexane to be completely removed, distill under reduced pressure...
Embodiment 2
[0032] The synthesis method of tetrakis (dimethylamino) hafnium, the steps are: under argon atmosphere, add 108 grams of dimethylamine and 200 mL of n-hexane into a 2000 mL three-necked bottle and stir evenly, and place the reaction bottle at -40 °C , according to the molar ratio of dimethylamine:n-butyllithium of 1.1:1, the n-butyllithium solution added dropwise to the reaction flask, stirred and reacted for 10 hours after the dropwise addition; according to hafnium tetrachloride:n-butyllithium The molar ratio is 1:4.1, add hafnium tetrachloride to the above reaction system, keep the temperature of the reaction system at 20°C, after adding hafnium tetrachloride, let the reaction system stir and react under the protection of inert gas 24 hours; after the reaction, remove the reaction solvent under an atmospheric pressure, wait for the solvent n-hexane to be completely removed, distill under reduced pressure, and collect the fraction at 80-85 °C / 2-5 mmHg, which is tetradimethyla...
Embodiment 3
[0034] The synthesis method of tetrakis (dimethylamino) hafnium, the steps are: under argon atmosphere, add 108 grams of dimethylamine and 200 mL of n-hexane into a 2000 mL three-necked bottle, stir evenly, and place the reaction bottle at -80 ℃, according to the molar ratio of dimethylamine:n-butyllithium of 1.2:1, add n-butyllithium solution dropwise to the reaction bottle, and stir for 10 hours after the addition; according to hafnium tetrachloride: n-butyl The molar ratio of lithium is 1:4.2, add hafnium tetrachloride to the above reaction system, keep the temperature of the reaction system at 60°C, after adding hafnium tetrachloride, let the reaction system stir under the protection of inert gas Reaction for 30 hours; after the reaction, remove the reaction solvent under an atmospheric pressure, wait for the solvent n-hexane to be completely removed, distill under reduced pressure, collect the fraction at 80-85°C / 2-5 mmHg, which is tetradimethylamino hafnium compound. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com