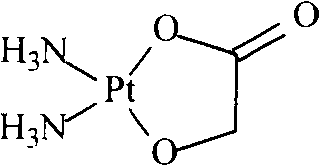
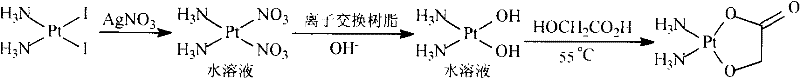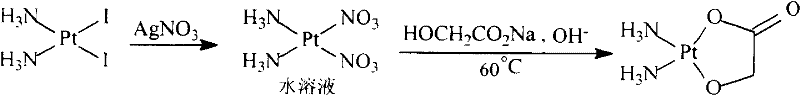Preparation method of nedaplatin with extremely low silver content
A nedaplatin and extremely low technology, applied in the field of nedaplatin preparation, can solve the problems of difficulty in accurately grasping the amount of potassium iodide used, silver ions cannot be completely removed, and it is not conducive to the removal of silver ions, etc. Stable, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 19.32 g (0.04 mol) of cis-diiododiammine platinum and 90 ml of deionized water into the reaction flask, and add a solution made of 13.59 g (0.08 mol) of silver nitrate (0.08 mol) and 50 ml of deionized water under stirring, and keep away from light at 25°C The reaction was stirred for 5h. The yellow silver iodide solid was filtered off to obtain a bright yellow filtrate (cis diammine platinum nitrate solution).
[0029] Put the filtrate in a 200mL beaker, insert the anode (graphite column, Φ0.8×4.0cm) and the cathode (stainless steel plate, 3.0×4.0cm), the pole distance is 1cm, adjust the electrolysis voltage to 1.75 volts, and the current to 20mA, power on and electrolyze 2h (until there is no significant increase in the black deposits deposited on the stainless steel plate). HPLC tracking showed that there was no significant change in the main components and impurities of the reaction solution before and after electrolysis.
[0030] After filtering, add 3.92 g o...
Embodiment 2
[0033] Add 19.32 g (0.04 mol) of cis-diiododiammine platinum and 90 ml of deionized water into the reaction bottle, add a solution made of 13.59 g (0.08 mol) of silver nitrate (0.08 mol) and 50 ml of deionized water under stirring, and keep away from light at 20°C The reaction was stirred for 6h. The yellow silver iodide solid was filtered off to obtain a bright yellow filtrate (cis diammine platinum nitrate solution).
[0034] The filtrate was placed in a 200mL beaker, the electrolysis device was the same as in Example 1, the electrolysis voltage was adjusted to 1.50 volts, the current was 10 milliamps, and the electrolysis was performed for 3 hours (the sediment deposited on the stainless steel plate did not increase significantly). HPLC tracking showed that there was no significant change in the main components and impurities of the reaction solution before and after electrolysis.
[0035] After filtering, add 3.92 g of sodium glycolate (0.04 mol) to the filtrate, adjust t...
Embodiment 3
[0038]Add 19.32 g (0.04 mol) of cis-diiododiammine platinum and 90 ml of deionized water into the reaction bottle, add a solution made of 13.59 g (0.08 mol) of silver nitrate (0.08 mol) and 50 ml of deionized water under stirring, and keep away from light at 25°C The reaction was stirred for 5h. The yellow silver iodide solid was filtered off to obtain a bright yellow filtrate (cis diammine platinum nitrate solution).
[0039] Add 3.92 g of sodium glycolate (0.04 mol) to the filtrate, adjust the pH to 7.0 with 2M sodium hydroxide solution, heat at 60° C., and stir for 6 hours. Concentrate under reduced pressure, crystallize, filter, wash the filter cake with a small amount of ice water, and dry to obtain 6.51 g of nedaplatin, with a yield of 53.7%.
[0040] Detected by atomic absorption spectrometry, the silver content in nedaplatin is 83ppm; analyzed by high performance liquid chromatography, the content of nedaplatin is 99.11%, and related substances <1.00%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com