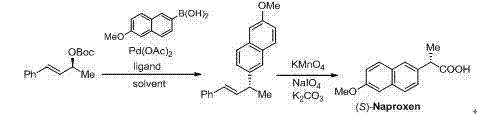
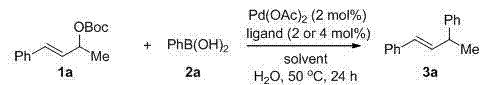Preparation method of 1,3-disubstituted-3-aryl propylene compound and application thereof
A technology for aryl propylene and compounds, which is applied in the field of preparation and application of 1,3-disubstituted-3-aryl propylene compounds, can solve the problems of lack of high efficiency and high selectivity, and achieve easy conversion, derivatization, and application Broad, Accessible Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
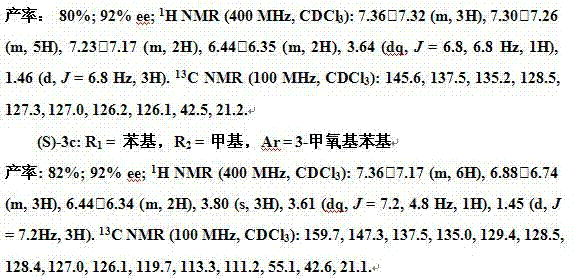
[0025] Embodiment 1: allyl carbonate compound and phenylboronic acid in Pd (OAc) 2 Allyl-aryl coupling reaction catalyzed by complex ligands and solvents
[0026] The allyl-aryl coupling reaction formula is as follows:
[0027]
[0028] Among them: mol is mole, ligand is ligand, solvent is solvent, Boc is tert-butoxycarbonyl, 2 mol% is used for bisphosphine ligand, and 4 mol% is used for monophosphine ligand.
[0029] As shown in Table 1, the allyl carbonate compounds and phenylboronic acid in Pd(OAc) 2 Allyl-aryl coupling reaction catalyzed by the complex ligand and organic solvent, and the corresponding yield:
[0030] Table 1
[0031]
[0032] Wherein, THF is tetrahydrofuran, and Toluene is toluene
[0033]
[0034] Of course, in this embodiment, the organic solvent can also be dioxane, methylene chloride, chloroform, benzene, ether, methanol, ethanol, isopropanol, n-butanol, tert-butanol, dimethyl Formamide or acetonitrile, etc., which can be realized by ...
Embodiment 2
[0035] Embodiment 2: Allyl carbonate compound 1a and phenylboronic acid (2a) in Pd(OAc) 2 Allyl-aryl coupling reaction catalyzed by the complex
[0036] As shown in Table 2, the allyl carbonate compound 1a and phenylboronic acid (2a) in Pd(OAc) 2 Allyl-aryl coupling reaction under the catalysis of the complex, the distribution ratio of ligands and solvent components, and the corresponding yields, wherein: the reaction solvent is tetrahydrofuran, the reaction temperature is 50 ° C, and the reaction time is 24 hours (where The reaction time of No. 7 is 48 hours).
[0037] Table 2
[0038]
Embodiment 3
[0039] Embodiment 3: Allyl carbonate compound 1a and phenylboronic acid (2a) in Pd (OAc) 2 The reaction temperature and time of the allyl-aryl coupling reaction catalyzed by the complex
[0040] As shown in Table 3, the allyl carbonate compound 1a and phenylboronic acid (2a) in Pd(OAc) 2 The reaction time, temperature and corresponding yield of the allyl-aryl coupling reaction catalyzed by the complex, where: 1a, 2a, Pd(OAc) 2 、PPh 3 The molar ratio with water is: 1:1.5:0.02:0.04:5; the reaction solvent is tetrahydrofuran.
[0041] table 3
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com