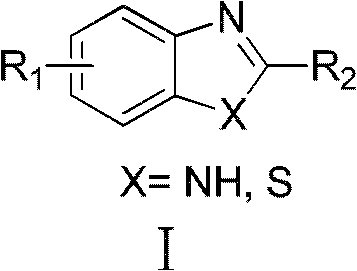
Method for closed-loop synthesis of benzoglioxaline and benzothiazole compounds by catalytic oxidation of primary alcohol
A technology of benzimidazole and benzothiazole, applied in the field of organic synthesis, which can solve the problems of environmental pollution, low yield, cumbersome post-processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
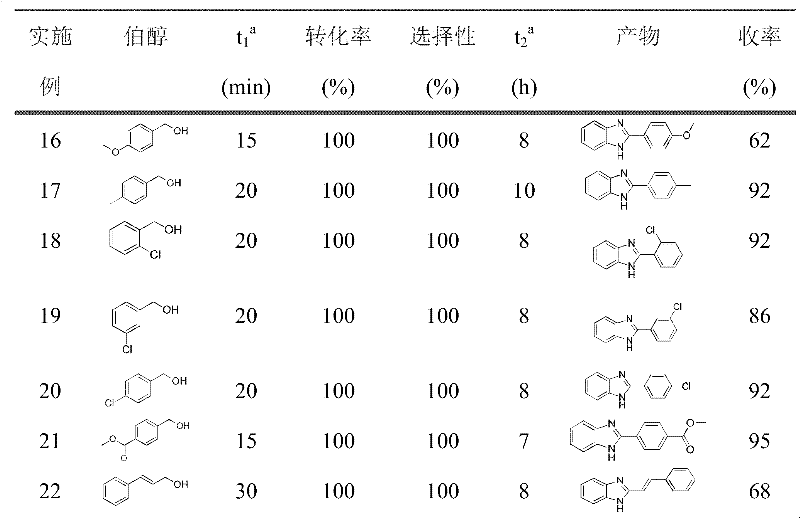
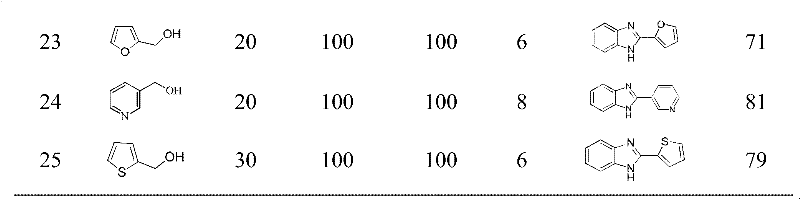
Examples
Embodiment 1
[0027] Synthesis of 2-phenyl-1H-benzimidazole
[0028] The reaction was carried out in a 316L stainless steel kettle (100 mL) with a Teflon lining equipped with a magnet, and the air in the kettle was not replaced by oxygen before the reaction. First add 10mL dichloromethane into the autoclave, then add 10.0mmol benzyl alcohol and 0.1mmol TEMPO into 10mL solvent, and then add 0.15mmol iron nitrate [Fe(NO 3 ) 3 ·9H 2 O], sealed. The autoclave was filled with air pressure to 0.8 MPa, and transferred to an oil bath that had been pre-heated to 80° C. After reacting for 0.5 hours, the stirring was stopped, and the pressure was reduced to room temperature. Sampling analysis adopts Agilent 7890N gas chromatograph, HP-5 gas chromatographic column (30m×0.25mm), FID detector, the detector temperature is 300℃, the inlet temperature is 300℃, and the furnace temperature is programmed to increase: 70℃ maintain After 2 minutes, the temperature was raised to 300°C at 10°C / min and held at 300°C ...
Embodiment 2
[0032] The test method and procedure are the same as in Example 1, but the transition metal salt used is copper nitrate [Cu(NO 3 ) 2 ·2.5H 2 O], the reaction temperature is 80 ℃, the first step of reaction is 1 hour after benzyl alcohol is completely converted into benzaldehyde, then o-phenylenediamine is added to it, the reaction is 6 hours, the yield of 2-phenyl-1H-benzimidazole 92%.
Embodiment 3
[0034] The test method and procedure are the same as in Example 1, but the transition metal salt used is chromium nitrate [Cr(NO 3 ) 3 ·9H 2 O], the reaction temperature is 80 ℃, the first step of reaction is 1 hour after benzyl alcohol is completely converted into benzaldehyde, then o-phenylenediamine is added to it, the reaction is 6 hours, the yield of 2-phenyl-1H-benzimidazole 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com