Thermal condensation production method for phenyl chlorosilane
A technology of phenylchlorosilane and a production method, which is applied in the field of production technology of phenylchlorosilane, can solve the problems of low production efficiency, material punching or explosion, stay and the like, and achieves the advantages of reducing energy consumption, reducing pollution and reducing reaction temperature. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
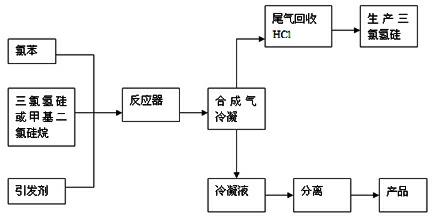
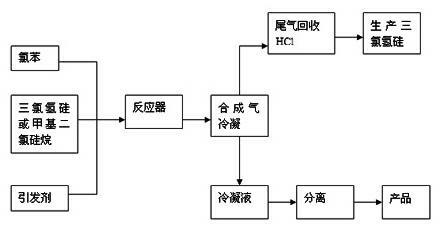
[0018] Such as figure 1 As shown, the process flow for preparing phenylchlorosilane is as described in the figure above. Specific steps are as follows:
[0019] step one:
[0020] Synthetic reaction: using raw materials chlorobenzene and trichlorosilane as raw materials, the ratio of the two is 0.7:1 in the molar ratio of chlorobenzene and trichlorosilane, and at the same time under the action of the initiator dibenzoyl peroxide, The temperature is controlled at 300-350°C and the pressure is 0.2-0.8Mpa to carry out the reaction. The amount of initiator dibenzoyl peroxide is 0.1%wt of the total amount of material. The reaction produces phenylchlorosilane mixed monomers.
[0021] Its reaction principle is:
[0022] C 6 h 5 Cl+HSiCl 3 →C 6 h 5 SiCl 3 +HCl(1)
[0023] C 6 h 5 Cl+HSiCl 3 →C 6 h 6 + SiCl 4 (2)
[0024] Step two:
[0025] Condensation of syngas: Use suitable refrigerant (air, circulating water, water at 0°C) to condense syngas to greatly reduce ...
Embodiment 2
[0031] Such as figure 1 As shown, the process flow for preparing phenylchlorosilane is as described in the figure above. Specific steps are as follows:
[0032] step one:
[0033] Synthesis reaction: use chlorobenzene and methyldichlorosilane as raw materials, the ratio of the two is 0.8:1 molar ratio of chlorobenzene and methyldichlorosilane, and at the same time, under the action of the initiator azobisisobutyronitrile Under the control temperature of 350-400°C and the pressure of 0.2-0.8Mpa, the reaction is carried out. The amount of initiator azobisisobutyronitrile is 1.05%wt of the total amount of material. The reaction produces phenylchlorosilane mixed monomers.
[0034] Its reaction principle is:
[0035] C 6 h 5 Cl+HSiCl 3 →C 6 h 5 SiCl 3 +HCl(1)
[0036] C 6 h 5 Cl+HSiCl 3 →C 6 h 6 + SiCl 4 (2)
[0037] Step two:
[0038] Condensation of syngas: Use suitable refrigerant (air, circulating water, water at 0°C) to condense syngas to greatly reduce e...
Embodiment 3
[0044] Such as figure 1 As shown, the process flow for preparing phenylchlorosilane is as described in the figure above. Specific steps are as follows:
[0045] step one:
[0046] Synthetic reaction: use raw materials chlorobenzene and trichlorosilane as raw materials, the ratio of the two is 0.8:1 molar ratio of chlorobenzene and trichlorosilane, and at the same time, under the action of initiator potassium persulfate, the temperature is controlled at React at 400-500°C and pressure 0.2-0.8Mpa. The amount of initiator potassium persulfate is 1.2%wt of the total amount of material. The reaction produces phenylchlorosilane mixed monomers.
[0047] Its reaction principle is:
[0048] C 6 h 5 Cl+HSiCl 3 →C 6 h 5 SiCl 3 +HCl(1)
[0049] C 6 h 5 Cl+HSiCl 3 →C 6 h 6 + SiCl 4 (2)
[0050] Step two:
[0051] Condensation of syngas: Use suitable refrigerant (air, circulating water, water at 0°C) to condense syngas to greatly reduce energy consumption; use three-stag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com