Preparation method of substitute natural gas
A technology for replacing natural gas and raw gas, applied in chemical instruments and methods, gas fuels, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of reduced catalyst service life, high nickel loading, and low reaction space velocity and other problems, to achieve the effects of reducing one-time investment and operating costs, high methane space-time yield, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
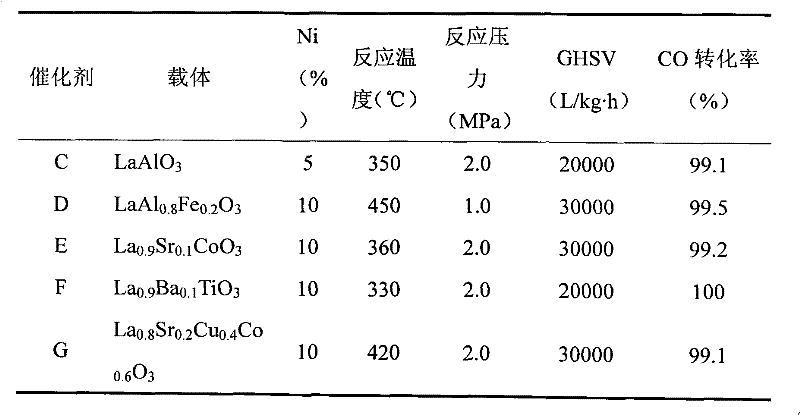
[0013] With the lanthanum-cobalt perovskite with a specific surface area of 70 square meters per gram as the carrier, the weight percentage of nickel oxide on the final catalyst is 10%, and the weight percentage of magnesium oxide as an auxiliary agent is 1.5%, using nickel nitrate and magnesium nitrate aqueous solution impregnation method Preparation of Ni-Mg / LaCoO 3 Catalyst (Catalyst A). Fill 5 grams of 20-40 mesh catalyst A in a quartz glass reaction tube with an inner diameter of 14 mm and a length of 250 mm, use a flow rate of 500 ml / min to contain 10% hydrogen / nitrogen mixed gas at 380 ° C for 6 hours, and then cool to room temperature , transferred to a stainless steel reactor with an inner diameter of 18 mm and a length of 300 mm. Raise the temperature of the reactor to 350°C, use the thermocouple inserted in the catalyst bed as the reaction temperature, and use syngas as the raw material to carry out the methanation reaction, where H 2 / CO (mol) = 3, CO 2 The co...
Embodiment 2
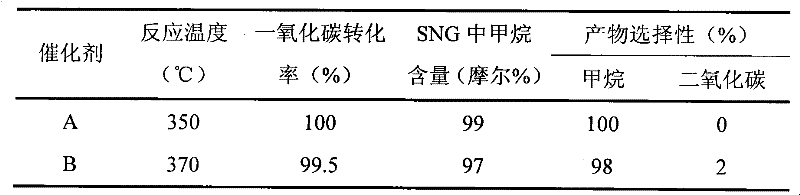
[0015] With the lanthanum-iron perovskite with a specific surface area of 30 square meters per gram as the carrier, the weight percent of nickel oxide on the final catalyst is 10%, and the weight percent of potassium oxide as an auxiliary agent is 1.0%, and the aqueous solution precipitation method of nickel nitrate and potassium nitrate is used Preparation of Ni-K / LaFeO 3 Catalyst (Catalyst B). According to the method described in Example 1, methanation is used to produce natural gas for replacement. The gas composition and catalyst performance after the reaction are listed in Table 1.
[0016] Table 1 Catalyst Evaluation Results*
[0017]
[0018] *GHSV=20000L / kg·h
Embodiment 3
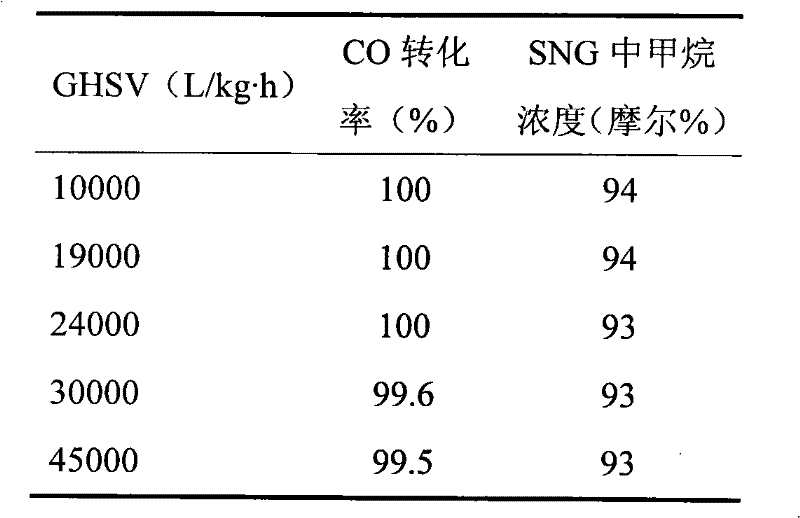
[0020] Using lanthanum-aluminum perovskite with a specific surface area of 20 square meters per gram as the carrier, the weight percent of nickel oxide on the final catalyst is 5%, and the weight percent of calcium oxide as an auxiliary agent is 2.0%, using nickel nitrate and calcium nitrate aqueous solution precipitation method Preparation of Ni-Ca / LaAlO 3 Catalyst (Catalyst C). H in mole percent 2 60%, CO 20%, CO 2 5%, CH 4 15% of the gas was used as a raw material for the synthesis of substituted natural gas. The catalyst loading was 30 g. After the reaction, the gas was subjected to pressure swing adsorption to obtain 95% methane mole percent of the substituted natural gas. The performance of the catalyst was listed in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com