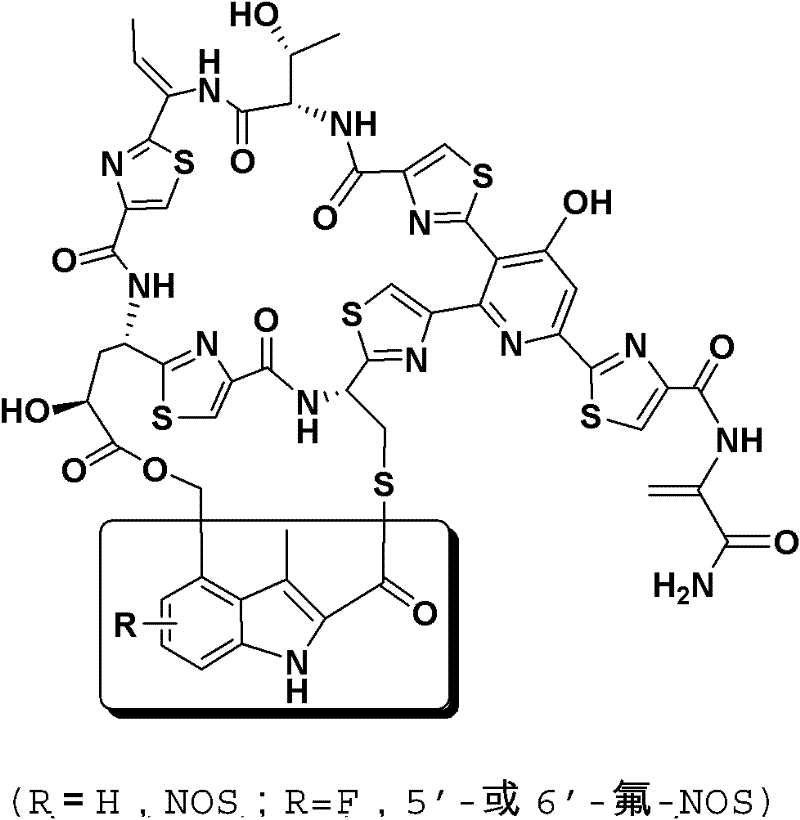
Novel fluoronosiheptide and preparation method and application thereof
A technology of flunoxetin and gnostomycin, which is applied in the field of precursor feeding to obtain fluorine-substituted gnostomycin, which can solve the problems of complex synthetic pathways and the failure to develop active gnostomycin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
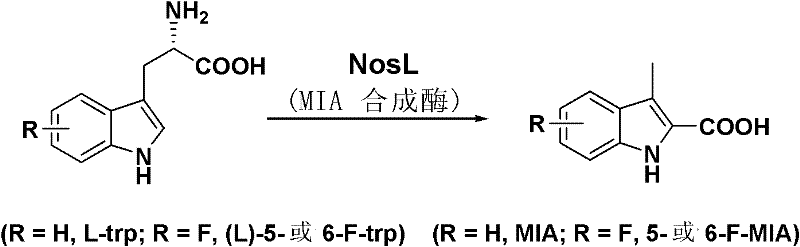
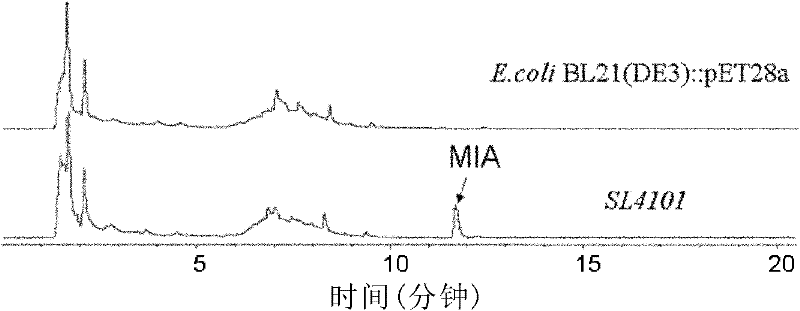
[0087] Example 1 The acquisition of Escherichia coli recombinant strain SL4101 and the preparation of MIA
[0088] In this example, the gene sequence encoding NosL in Streptomyces actuosus was amplified by PCR and ligated into the pET28a expression vector, and then the successfully constructed plasmid was introduced into E.coliBL21(DE3) to obtain the recombinant strain SL4101. The expression of NosL in SL4101 was induced for more than 5 hours, and a large amount of MIA (greater than 40 mg / l) was detected in the supernatant of the fermentation broth and bacteria.
[0089] 1. Construction of NosL expression strain SL4101
[0090] The primer sequences for cloning NosL are as follows:
[0091] NosL-forward: 5'-TTGAATTCATGACGCAGAACTCCCAGG-3' (SEQ ID NO: 1)
[0092] NosL-reverse: 5'-TTTAAGCTTTCAGACCGCCCGGGACGCCTC-3' (SEQ ID NO: 2)
[0093] Using the extracted total DNA of active Streptomyces as a template, PCR amplification was performed with the above-mentioned specific primers ...
Embodiment 2
[0109] The acquisition of embodiment 2 fluorinated MIA
[0110] In this example, the SL4101 bacteria expressed for 5 hours were resuspended in a mixed medium formed by mixing conventional LB medium and M9 medium at a ratio of 1:1, and fed with fluorine-substituted tryptophan (5- fluorotryptophan or 6-fluorotryptophan), cultivated for more than 5 hours, can obtain about 10 mg / l of corresponding fluorine-substituted MIA, and the compound is detected by LC / MS ( Figure 4 ), and after separation, purification and characterization, the structure was confirmed.
[0111] In addition, using a method similar to that of Example 1, the SL4101 bacterial solution stored at -80°C was streaked on an LB (kanamycin 50 μg / ml) plate and cultured overnight. Pick single colonies and inoculate them in 3ml LB medium (Kanamycin 50μg / ml) and incubate at 37°C for more than 8 hours, then inoculate in 800ml LB medium (Kanamycin 50μg / ml), and incubate at 37°C for 2.5 hours (A 600nm 0.5-0.7), transferred...
Embodiment 3
[0123] Embodiment 3 Fermentation, detection, separation and purification and activity determination of fludenoxetin
[0124] In this example, the fluorine-substituted tryptophan (5-fluorotryptophan or 6-fluorotryptophan) was fed to Nosiheptide-producing bacteria Streptomyces actuosus to obtain the corresponding fluorine-substituted Nosiella (5'-flunoxetin or 6'-flunoxetin). Methods as below:
[0125]Inoculate a single colony of S.actuosus, a gnosmycin-producing bacterium, into 3ml of conventional TSB medium, culture for 36-48 hours, and inoculate the bacterial liquid into 50ml of TSB medium until the logarithmic growth phase. At this time, inoculate 3-4ml of bacterial liquid into 50ml of pre-fermentation medium (glucose 5g, corn steep liquor 15ml, peptone 5g, yeast extract 1g, malt extract 1g, magnesium sulfate heptahydrate 0.2g, ammonium sulfate 1.5g, calcium carbonate 2.5g, pH=6.8), cultivated at 30°C for 28-36 hours until the cells grow dense. Inoculate 10ml of pre-ferme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com