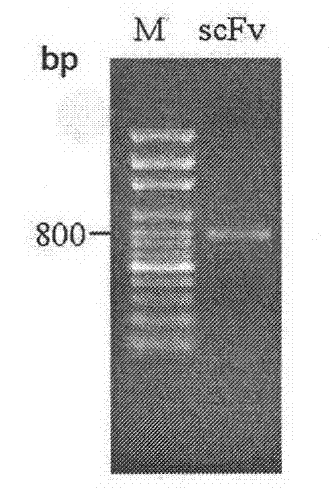
Screening and application of single-chain antibody against fumonisin
A fumonisin, single-chain antibody technology, applied in the field of genetic engineering, can solve the problems of unpublished nucleotide or amino acid sequence, high cost, time-consuming and laborious, etc., to improve stability or affinity, low production cost, expensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Antigen Preparation
[0043] One-step coupling with glutaraldehyde (Azcona-Olivera, J.I.; Abouzied, M.M.; Plattner, R.D.; Norred, W.P.; Pestka, J.J. Generation of antibodies reactive with fumonisins B 1 , B 2 , and B 3 by using cholera toxin as the carrier-adjuvant.Appl.Environ.Microbiol.1992,58,169-173.) Fumonisin FB1 (purchased from Sigma Company) and keyhole limpet cyanin (KLH, purchased from Sigma Company ) coupling (KLH-NH 2 +OHC-(CH 2 ) 3 -CHO+FB1-NH 2 → KLH-N=HC-(CH 2 ) 3 -CH=N-FB1) as an immune antigen (FB1-KLH), the fumonisin FB1 was coupled with bovine serum albumin (BSA, purchased from Sigma) (BSA-NH 2 +OHC-(CH 2 ) 3 -CHO+FB1-NH 2 →BSA-N=HC-(CH 2 ) 3 -CH=N-FB1) was used as the screening antigen (FB1-BSA).
Embodiment 2
[0044] Example 2: Animal immunization
[0045] Two BALB / c mice (Experimental Animal Center, Wuhan Institute of Virology, Chinese Academy of Sciences) were immunized with the prepared antigen FB1-KLH 4 times with an interval of 10 days between each time. For the first time, 250 μl of immune antigen was mixed with an equal volume of Freund's complete adjuvant for immunization, and for the last three times, 250 μl of immune antigen was mixed with an equal volume of Freund's incomplete adjuvant for immunization. On the 5th day after the third immunization, the serum was collected from 1:10 3 Doubling diluted to 1:128×10 3 , with reference to the indirect ELISA method introduced by Lin Qiaoai and Dong Haiyan, "Experimental Guidance for Medical Immunology and Microbiology", Zhejiang University Press, 2006, to detect the level of antibodies in the serum of immunized mice. The test results showed that the serum of immunized mice produced higher antibody titers (dilution > 1:10 5 )....
Embodiment 3
[0046] Example 3: Spleen RNA Extraction, mRNA Purification and cDNA First-Strand Synthesis of Immunized Mice
[0047] 1) The immunized mice with higher antibody titers were given a booster immunization once more, and the spleens of the immunized mice were taken 7 days later.
[0048] 2) Using TRNzol-A + Total RNA extraction reagent (purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd., operated according to the instructions of the reagent) was used to extract total RNA from spleen.
[0049] 3) Purify the total RNA extracted in step 2) with an mRNA purification kit (purchased from QIAGEN, and operate according to the instructions of the kit).
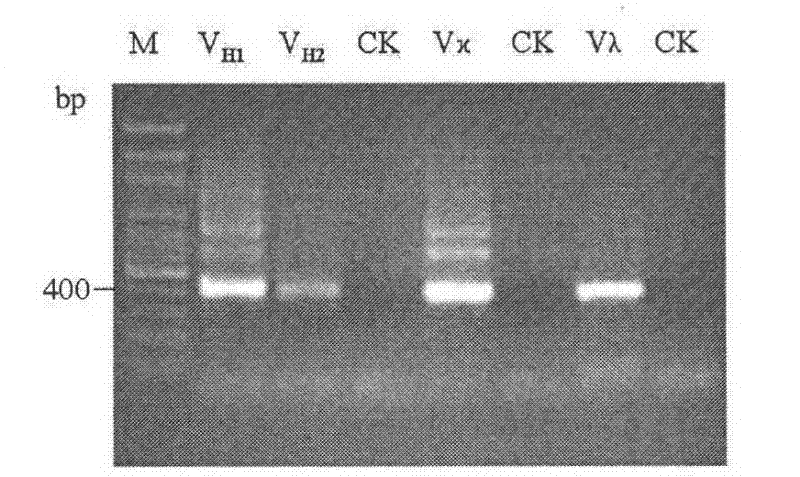
[0050] 4) Use SuperScript TM III Reverse transcriptase (purchased from Invitrogen Company, operated according to the instructions of the enzyme) reverse-transcribed the purified mRNA obtained in step 3), and synthesized antibody heavy chain (IgG 1 and IgG 2a / 2b ) and light chain (kappa chain and lambda chain) variable r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com