Process for production of organic acids
A technology of tartaric acid and glycolic acid, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve the problems of low yield and cannot be prepared with glycolic acid, etc. achieve high practicality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
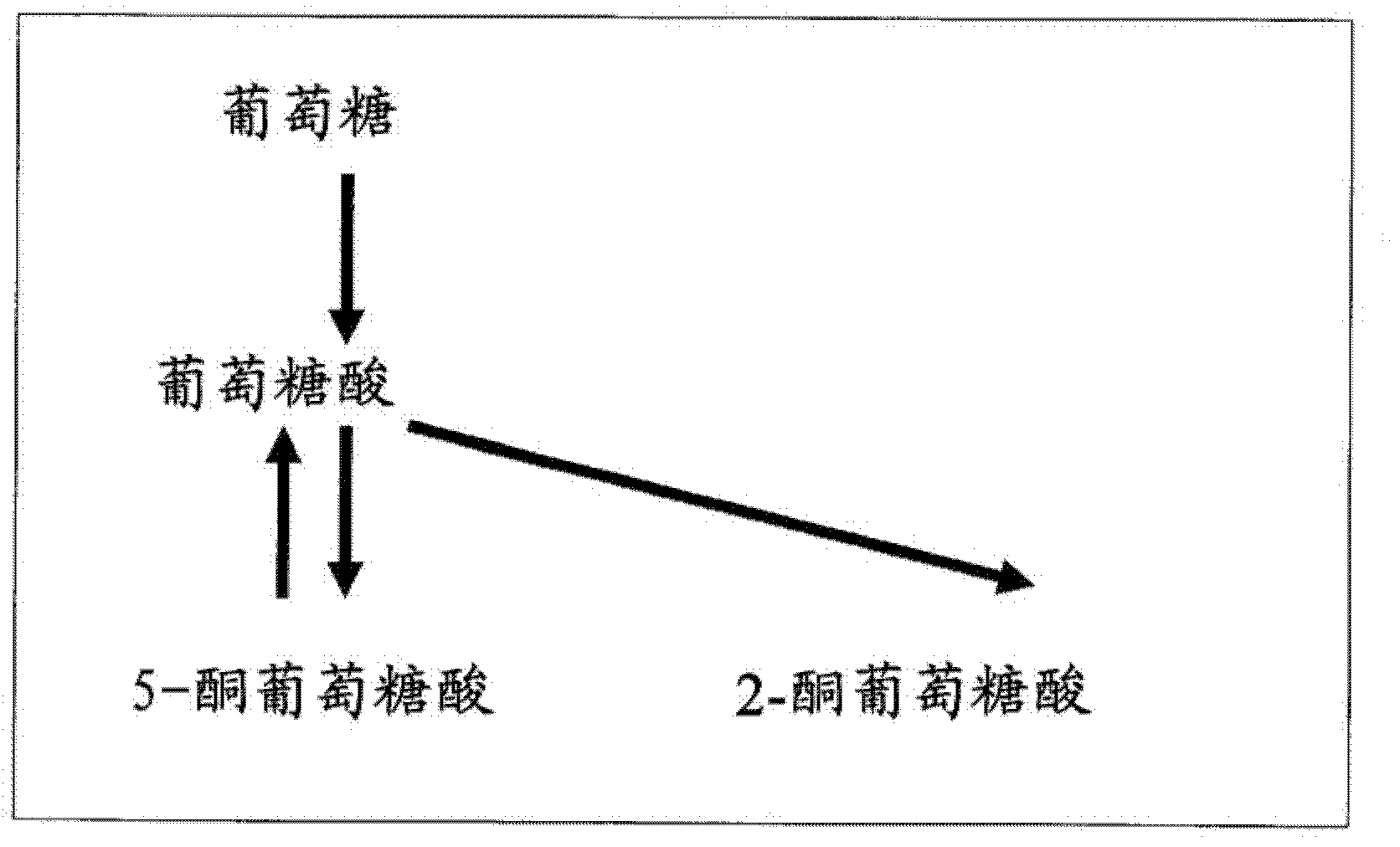
[0052] [Acquisition of 2-keto-D-gluconic acid non-producing bacteria and 5-keto-D-gluconic acid non-consuming mutant strains]
[0053] In order to prepare a 2-keto-D-gluconic acid non-producing strain derived from the Gluconobacter oxydans NBRC 3172 strain, an NTG mutation treatment was performed on the NBRC 3172 strain, and screening of 2-keto-D-gluconic acid non-producing bacteria was attempted. Specifically, it was carried out by the following method.
[0054] Oxidation cells grown on MA agar medium [2.5% mannitol, 0.5% yeast extract (manufactured by Difuco Laboratori Co., Ltd.), 0.2% peptone (manufactured by Diifco Laboratori Co., Ltd.), 1.5% agar (no pH adjustment)] Gluconobacter NBRC 3172 strain was inoculated with 1 platinum circle into a 300-mL Erlenmeyer flask containing 30 mL of MA medium sterilized in an autoclave at 121°C for 20 minutes, and cultured with shaking at 220 rpm per minute at 30°C After 19 hours, cells were aseptically collected from the culture soluti...
Embodiment 2
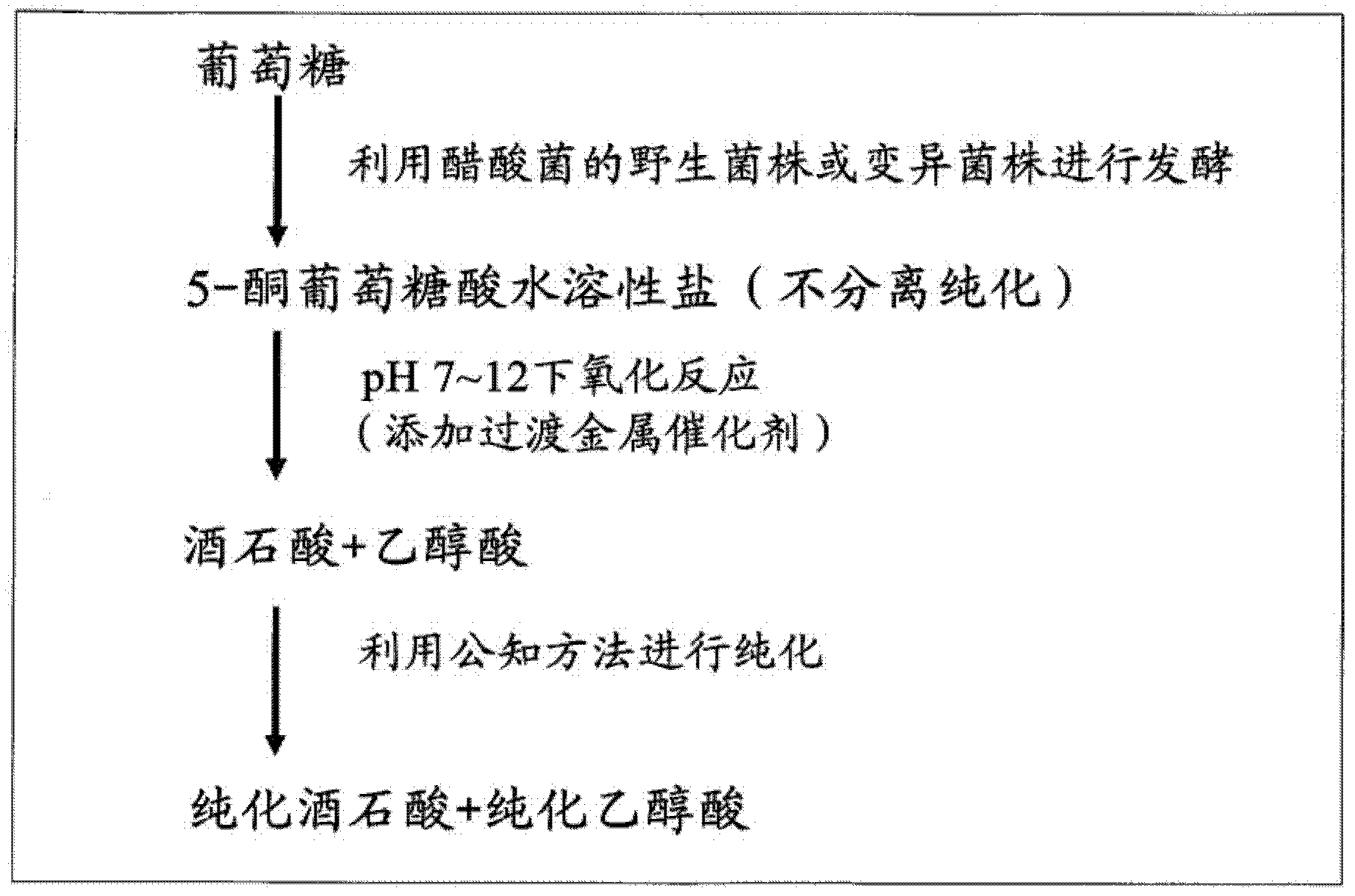
[0058] [(Preparation of Tartaric Acid and Glycolic Acid from Glucose (Addition of Transition Metal Catalyst))]
[0059] The Gluconobacter oxidans TADK-267 strain obtained in the above Example 1 was inoculated into 5 mL of MB medium [2.5% mannitol, 0.5% yeast extract (Difuco Laboratori Co., Ltd. ) and 0.3% peptone (manufactured by Difuco Laboratori Co., Ltd.)], and cultured at 28° C. for 17 hours with reciprocating shaking at 250 rpm. The culture solutions in the three test tubes were combined as the seed culture solution. This culture is carried out as follows. In a 1L fermenter (manufactured by Eibul Corporation), add 6% glucose, 0.09% ammonium chloride, 0.06% potassium dihydrogen phosphate, 0.18% corn steep liquor dry powder (manufactured by Sigma), 0.1% Yeast extract (manufactured by Difuko Laboratori Co., Ltd.), 0.015% magnesium sulfate heptahydrate, 0.0029% manganese sulfate pentahydrate, 0.12% calcium chloride dihydrate, and 0.1% Actocol (manufactured by Takeda Chemical...
Embodiment 3
[0061] [Preparation of tartaric and glycolic acids from glucose (adding palladium on carbon)]
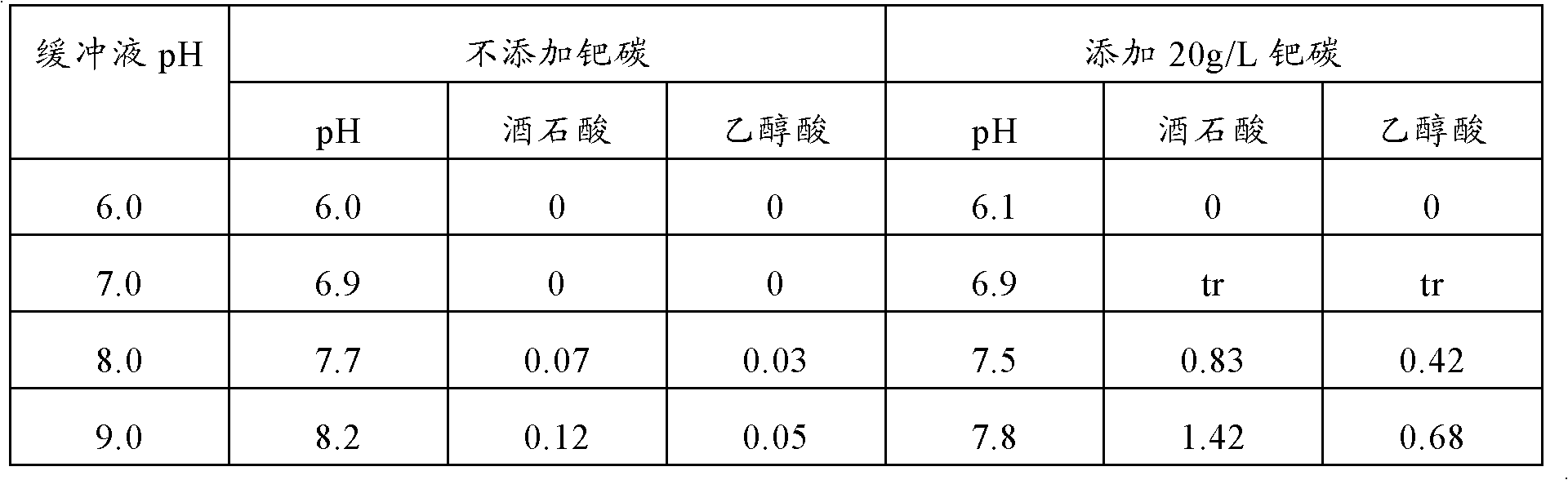
[0062]After cultivating Gluconobacter oxydans TADK-267 in a fermenter for 50 hours in the same manner as in Example 2 above, glucose was completely consumed and 2.8 g / L of gluconic acid remained, but 67.4 g / L of 5-keto- D-gluconic acid. After adding 6M potassium hydroxide solution to the culture solution to raise the pH of the culture solution to 9.6, 10 g of palladium activated carbon (10%, manufactured by Wako Pure Chemical Industries, Ltd.) was added, and then potassium hydroxide solution was added to maintain the pH at At the same time as above 9.6, continue aeration and stirring until after the 144th hour, a reaction solution containing 31.9 g / L of tartaric acid and 15.9 g / L of glycolic acid is obtained. If considering the change of the liquid volume caused by evaporation and adding potassium hydroxide solution, and the liquid volume reduction caused by sampling in the middle ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com