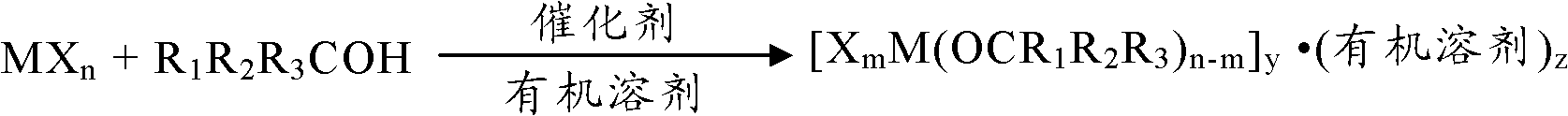Metallic alkoxy complex, catalyst composition and preparation method of poly-caprolactone or poly-lactide
A technology of metal alkoxy and complexes, applied in metal/metal oxide/metal hydroxide catalysts, preparation of organic compounds, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of polylactide Low molecular weight, wide molecular weight distribution, and uncontrollable molecular weight of polyε-caprolactone, etc., to achieve high catalytic activity, reduced dosage, and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The present invention also provides the preparation method of polyε-caprolactone and polylactide, comprising the following steps:
[0062] The ε-caprolactone monomer or lactide monomer reacts under the action of a catalyst to obtain poly ε-caprolactone or polylactide, and the catalyst is the metal alkoxy complex or A catalyst composition composed of a metal alkoxy complex and a compound containing a hydroxyl group.
[0063] The molar ratio of the metal alkoxy complex to the ε-caprolactone monomer or lactide monomer in the catalyst is 1:1-10000, preferably 1:1000-9000.
[0064] The polymerization reaction time of ε-caprolactone monomer or lactide monomer is preferably 0.03h~24h, more preferably 0.5h~20h, most preferably 3h~10h; ε-caprolactone monomer or lactide The temperature of monomer polymerization reaction is preferably 10°C to 130°C, more preferably 25°C to 100°C.
[0065] Since metal alkoxy complexes are sensitive to oxygen and water, the reaction is preferably ...
Embodiment 1
[0070] Embodiment 1 prepares metal alkoxy complexes A1~A9
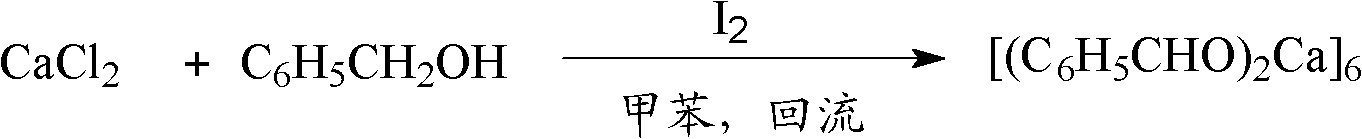
[0071] Under anhydrous and oxygen-free conditions, slowly drop 10mL of toluene solution containing benzyl alcohol into the toluene solution containing calcium chloride, wherein the content of benzyl alcohol is 0.22g (2mmol), and the content of calcium chloride is 0.11g (1mmol); Add 0.017g elemental iodine, reflux reaction 24h, reaction formula is as follows:
[0072]
[0073] The reaction mixture was cooled and filtered to obtain 0.25 g of white solid complex A1 with a yield of 76%.
[0074] The complex A1 was subjected to elemental analysis, and its molecular formula was C 14 h 12 CaO 2 , molecular weight 252.32: C, 66.64; H, 4.79; Ca, 15.88; O, 12.68.
[0075] The preparation method of complexes A2~A9 is the same as that of A1, the difference is that methanol, ethanol, isopropanol, n-butanol, tert-butanol, phenol, diphenylmethanol and triphenylmethanol are reacted with calcium chloride in sequence , were obt...
Embodiment 2
[0084] Example 2 Preparation of metal alkoxy complexes A10-A18
[0085] Under anhydrous and oxygen-free conditions, 10 mL of toluene solution containing benzyl alcohol is slowly added dropwise to the toluene solution containing bistrimethylsilylamide magnesium, wherein the content of benzyl alcohol is 0.22 g (2 mmol), bistrimethylsilylamide The content of magnesium methylsilylamide is 0.11g (1mmol); 0.017g elemental iodine is added, and the reflux reaction is carried out for 24h. The reaction formula is as follows:
[0086]
[0087] The reaction mixture was cooled and filtered to obtain 0.38 g of white solid complex A10 with a yield of 90%.
[0088] The complex A10 was subjected to elemental analysis, and its molecular formula was C 14 h 12 MgO 2 , with a molecular weight of 236.5: C, 71.08; H, 5.11; Mg, 10.27; O, 13.53.
[0089] The preparation method of complexes A11~A18 is the same as that of A10, the difference is that methanol, ethanol, isopropanol, n-butanol, tert...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com