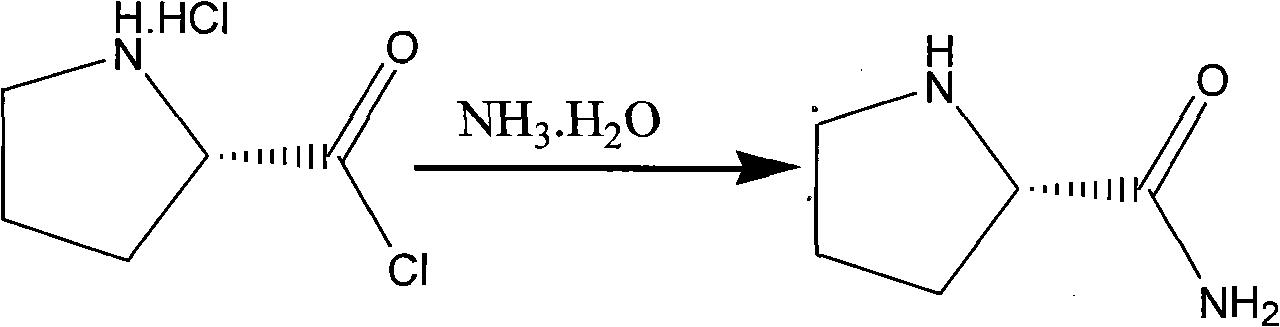Method for preparing (2S)-N-chloracetyl-2-cyano-group pyrrolidine
A technology of cyanotetrahydropyrrole and formamidotetrahydropyrrole is applied in the field of preparing -N-chloroacetyl-2-cyanotetrahydropyrrole, and can solve the problem of difficult industrialized production, high cost, low yield, etc. problem, to achieve the effect of less difficulty in operation, improved yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: a kind of preparation (2S)- N -Chloroacetyl-2-cyanotetrahydropyrrole method
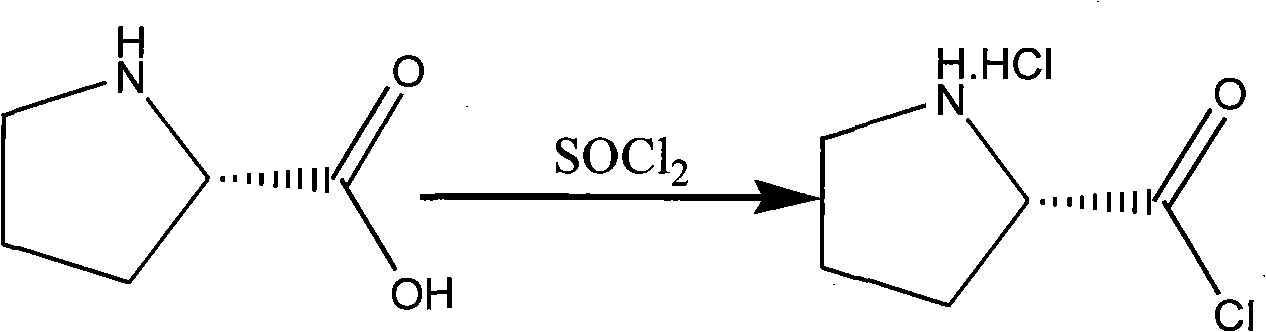
[0026] (1) Add 10g of L-proline and 50ml of dichloromethane into a 100ml four-neck flask, start stirring, cool down to 0-5°C, maintain this temperature and slowly add 20.7g of thionyl chloride dropwise, and heat up to Reflux and keep warm for 1 hour. After the reaction is complete, the solvent is evaporated to dryness under reduced pressure at 30° C. to obtain 15 g of L-prolyl chloride.
[0027] (2) Slowly add the above-mentioned 15gL-prolyl chloride dropwise to 100g 20% ammonia water at 0-5°C, and react at -10°C-40°C for 1-10 hours. After the reaction is complete, dichloromethane 100ml*6 was extracted six times, the organic layers were combined, dried, suction filtered, and the filtrate was precipitated under reduced pressure at 30°C to dryness to obtain 8g of L-prolinamide, Mp: 96~98°C, [α]D 20 -106 0 (c=2C 2 h 5 Oh)
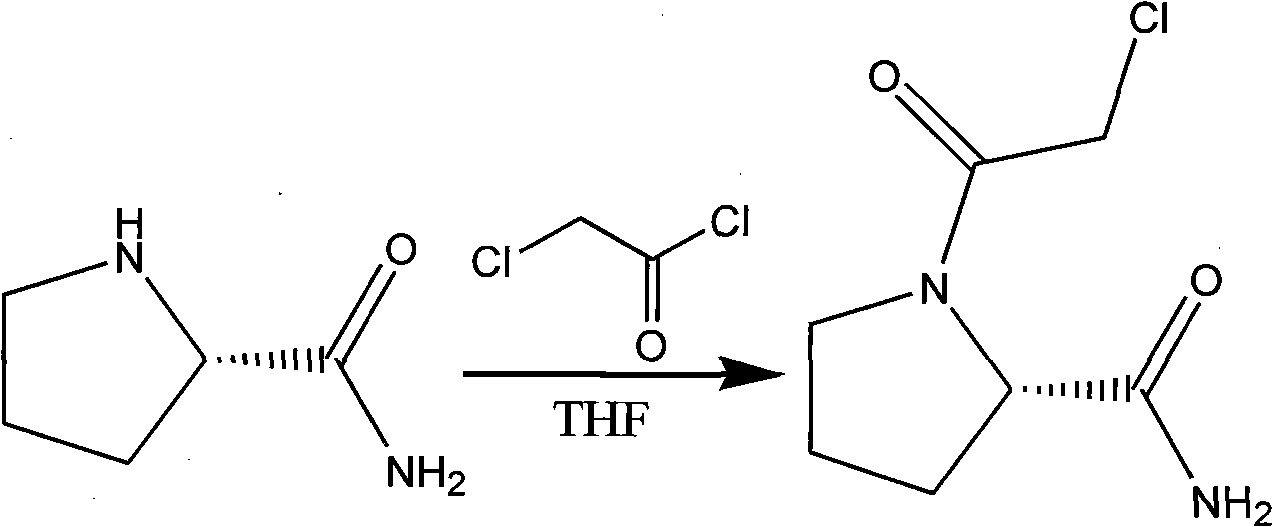
[0028] (3) Add 100ml of tetrahydrofuran to 8gL-pr...
Embodiment 2
[0033] Embodiment 2: a kind of preparation (2S)- N -Chloroacetyl-2-cyanotetrahydropyrrole method
[0034] The process of this embodiment is the same as that of Example 1, and the difference is that the heat preservation and reflux in the step of preparing prolyl chloride in the step (1) is changed from 1 hour to 10 hours, and the yield can still be 15gL-prolyl chloride, and thus As a raw material does not affect the next step of the reaction.
Embodiment 3
[0035] Embodiment 3: A kind of preparation (2S)- N -Chloroacetyl-2-cyanotetrahydropyrrole method
[0036] The process of this embodiment is the same as that of Example 1, the difference being that the reaction temperature of adding prolyl chloride dropwise to ammonia water in step (2) is changed to 0°C~40°C, the reaction time is changed to 3~6 hours, and the The rate can still achieve 8gL-prolineamide and it will not affect the next step reaction as a raw material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com