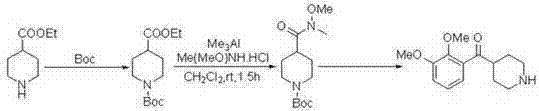
Preparation method of N-methoxy-N-methyl-1-p-toluenesulfonyl piperidine-4-amide
A technology of tosylpiperidine and methoxyamine, applied in the field of chemical synthesis, can solve the problems of difficult separation, inconvenient operation and high cost, and achieve the effects of simple post-processing process, shortened reaction period and simplified reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Synthesis of 1-tosylpiperidine-4-carboxylic acid
[0028] Piperidine-4-carboxylic acid (5g, 38.8mmol) and 1.67mol / L sodium hydroxide aqueous solution (3.1gNaOH, 46mlH 2 O) Add it to a 100ml reactor, cool to 0°C, slowly add TsCl in ether solution (7.4g, 38.8mmolTsCl, 40mlEt 2 O), stirred for 4 hours, evaporated the ether, and acidified it to pH = 2 with concentrated hydrochloric acid, immediately precipitated a large amount of white solid, and dried it in vacuum to obtain 1-tosylpiperidine-4-carboxylic acid with a yield of 99%.
[0029] The reaction equation is as follows:
[0030]
[0031] Melting point: 167~169°C.
[0032] 1 HNMR (400HZ, CDCl 3 )δ:2.46(s,3H),1,81~1.87(m,2H),1.99(d, J =3.2Hz,1H),2.26~2.30(m,1H),2.45~2.48(m,1H),7.33(d, J= 8.0Hz,2H); 13 CNMR (400HZ, CDCl 3 ): 21.512ppm, 27.182ppm, 39.833ppm, 45.343ppm, 76.680ppm, 77.000ppm, 77.312ppm, 127.633ppm, 129.676ppm, 132.915ppm, 143.653ppm, 179.822ppm.
[0033] (2) Synthesis of N-methoxy-N-meth...
Embodiment 2
[0040] (1) Synthesis of 1-tosylpiperidine-4-carboxylic acid
[0041] Same as embodiment one.
[0042] (2) Synthesis of N-methoxy-N-methyl-1-p-toluenesulfonylpiperidine-4-amide
[0043]Add 1-toluenesulfonylpiperidine-4-carboxylic acid (0.5g, 1.67mmol) and N-methyl-N-methoxylamine (0.3g, 5mmol) into a 100ml single-necked bottle, add 10ml of toluene, and cool to 0°C, then add PCl 3 (0.1g, 0.84mmol) of toluene solution was slowly added dropwise, stirred at 0°C for 4-5h, quenched with saturated sodium bicarbonate solution, extracted with ethyl acetate (4×3ml), and the extract was dried over anhydrous magnesium sulfate , filtered, and concentrated to give a white solid. The yield is 96%.
[0044] Synthetic N-methoxy-N-methyl-1-p-toluenesulfonylpiperidine-4-amide, via 1 HNMR, 13 CNMR detects that its product is a pure compound, and its performance indicators or characterization data are the same as in Example 1.
Embodiment 3
[0046] (1) Synthesis of 1-tosylpiperidine-4-carboxylic acid
[0047] Same as Example 1
[0048] (2) Synthesis of N-methoxy-N-methyl-1-p-toluenesulfonylpiperidine-4-amide
[0049] Add 1-toluenesulfonylpiperidine-4-carboxylic acid (0.5g, 1.67mmol) and N-methyl-N-methoxylamine (0.3g, 5mmol) into a 100ml single-necked bottle, add 10ml of dichloromethane , cooled to 0°C, and then the PCl 3 (0.1g, 0.84mmol) dichloromethane solution was slowly added dropwise, stirred at room temperature for 6-8h, quenched with saturated sodium bicarbonate solution, extracted with ethyl acetate (4×3ml), and the extract was washed with anhydrous magnesium sulfate Dry, filter, and concentrate to a white solid. The yield is 94%.
[0050] The above-mentioned synthetic N-methoxy-N-methyl-1-p-toluenesulfonylpiperidine-4-amide, through 1 HNMR, 13 CNMR detection, its product is a pure compound, its various performance indicators or characterization data Ketone Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com