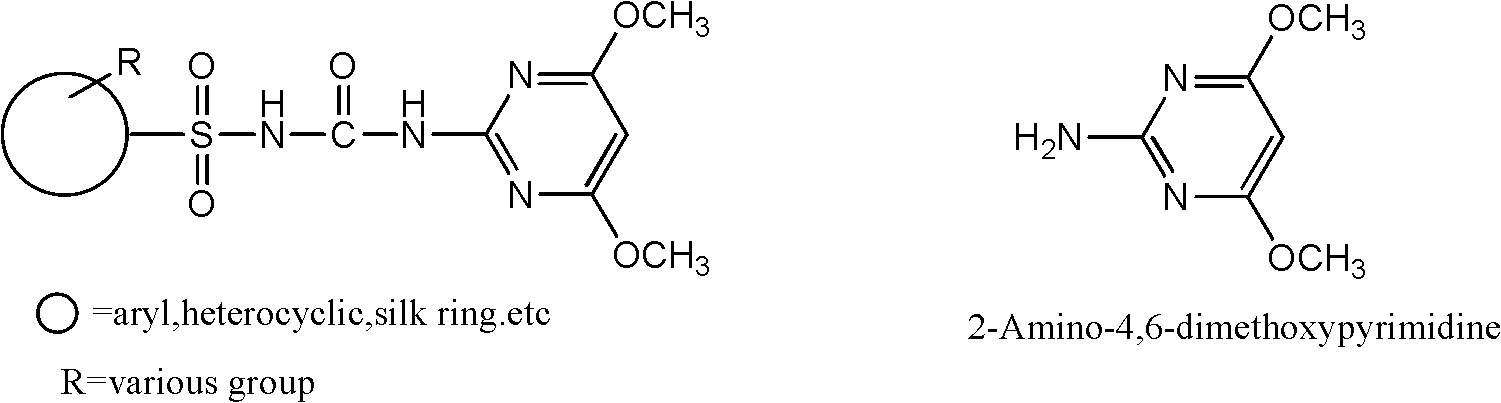
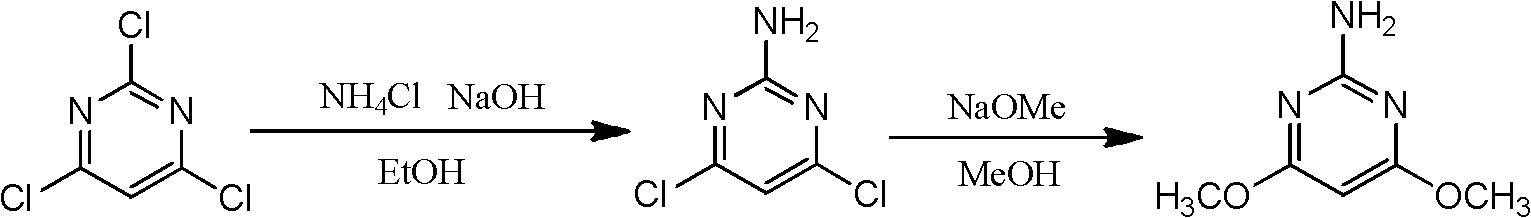
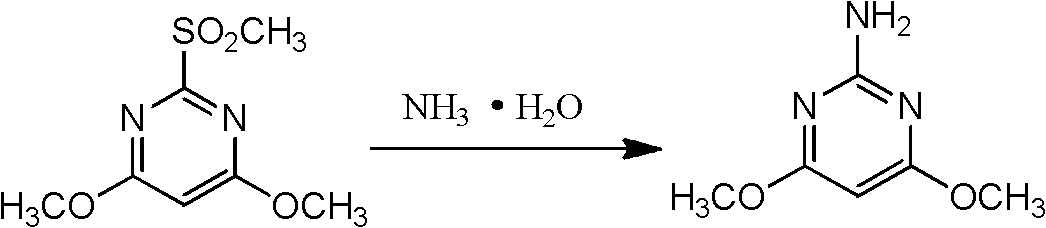Preparation method for 2-amino-4, 6-dimethoxy pyrimidine
A technology of dimethoxypyrimidine and amino, which is applied in the field of preparation of chemical intermediates, can solve the problems of unavailable raw materials of chlorobenzene, harm to people and the environment, and high price, and achieve the reduction of recrystallization links, easy availability of raw materials, high high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Addition reaction (preparation of 1,3-dimethoxypropanediimine dihydrochloride)
[0057] In a 3000L glass-lined kettle, add metered 1800L methyl acetate through the head tank, start stirring, turn on frozen brine, and cool down to below 0°C; open the vent valve of hydrogen chloride that has been dried, and introduce hydrogen chloride into the kettle, temperature control Below 10°C; when the pressure in the kettle rises to 0.03MPa, the temperature drops, the absorption of hydrogen chloride slows down, and the hydrogen chloride reaches saturation in 3 hours, and then the prepared malononitrile-methanol solution (200kg malononitrile and 270L Methanol, nitrogen gas is filled in the head tank, the pressure in the head tank is higher than the pressure in the kettle), the temperature is 5-10°C, after the dropwise addition (about 5 hours), it is reacted at 15-20°C for 3 hours, and the sampling is controlled in the center; the control is qualified Afterwards, use nitrogen gas ...
Embodiment 2
[0065] 1. Addition reaction (preparation of 1,3-dimethoxypropanediimine dihydrochloride)
[0066] In a 3000L glass-lined kettle, add metered 1800L methyl formate through the head tank, start stirring, turn on frozen brine, and cool down to below 0°C; open the vent valve of hydrogen chloride that has been dried, and introduce hydrogen chloride into the kettle, temperature control Below 10°C; when the pressure in the kettle rises to 0.03MPa, the temperature drops, the absorption of hydrogen chloride slows down, and the hydrogen chloride reaches saturation in 4 hours, and then the prepared malononitrile-methanol solution (200kg malononitrile and 270L Methanol, filled with nitrogen in the head tank, the pressure in the head tank is higher than the pressure in the kettle), the temperature is 5°C, the dropwise addition is completed and reacted at 15°C for 3 hours, and the sampling is controlled in the center; after the control is qualified, press filter, vacuum -0.08MPa and Dry at a...
Embodiment 3
[0074] 1. Addition reaction (preparation of 1,3-dimethoxypropanediimine dihydrochloride)
[0075] In a 3000L glass-lined kettle, add metered 2200L methyl acetate mother liquor (solvent applied separately) through the head tank, start stirring, turn on frozen brine, and cool down to below 0°C; open the dried hydrogen chloride ventilation valve, and vent Add hydrogen chloride, and the temperature is controlled below 10°C; when the pressure in the kettle rises to 0.03MPa, the temperature decreases, the absorption of hydrogen chloride slows down, and the hydrogen chloride reaches saturation in 1.5 hours, then dropwise add the prepared malononitrile-methanol solution (200kg Malononitrile and 250L methanol, nitrogen is filled in the head tank, the pressure in the head tank is higher than the pressure in the kettle), the temperature is 10°C, the dropwise addition is completed and reacted at 15°C for 3 hours, and the sampling is controlled in the middle; after the middle control is qua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com