Quinoxaline derivative and preparation method thereof
A kind of technology of quinoxaline and derivatives, applied in the field of quinoxaline derivatives and preparation thereof, can solve the problems such as the limitation of types of dihydrophenazine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] In the preparation method provided by the present invention, the raw materials and reagents used are known, which can be purchased in the market, or prepared according to the prior art. For example, the preparation of N,N'-diaryl-o-phenanthrene diamine compounds can be synthesized by referring to the method reported in the literature (J.Chem.Soc.D, 1970, 1552-1553).
[0035] In the preparation method provided by the present invention, the reaction temperature of the N,N'-diaryl-phenanthrene diamine compound and the halogenated aromatic hydrocarbon is 20°C-250°C, more preferably 150°C-230°C. The reaction time is at least 1 hour, preferably the reaction time is 1 hour to 48 hours. The molar ratio of N,N'-diaryl-o-phenanthrene diamine compound to halogenated aromatic hydrocarbon is preferably 1:(0.5-2).
[0036] Catalyst used in the present invention is selected from: copper, palladium, platinum, copper-containing compound, palladium-containing compound or the mixture of ...
Embodiment 1
[0042] Synthesis of 1,6-diphenyl-benzo[b]-dibenzo[f,h]quinoxaline (compound I a)
[0043]
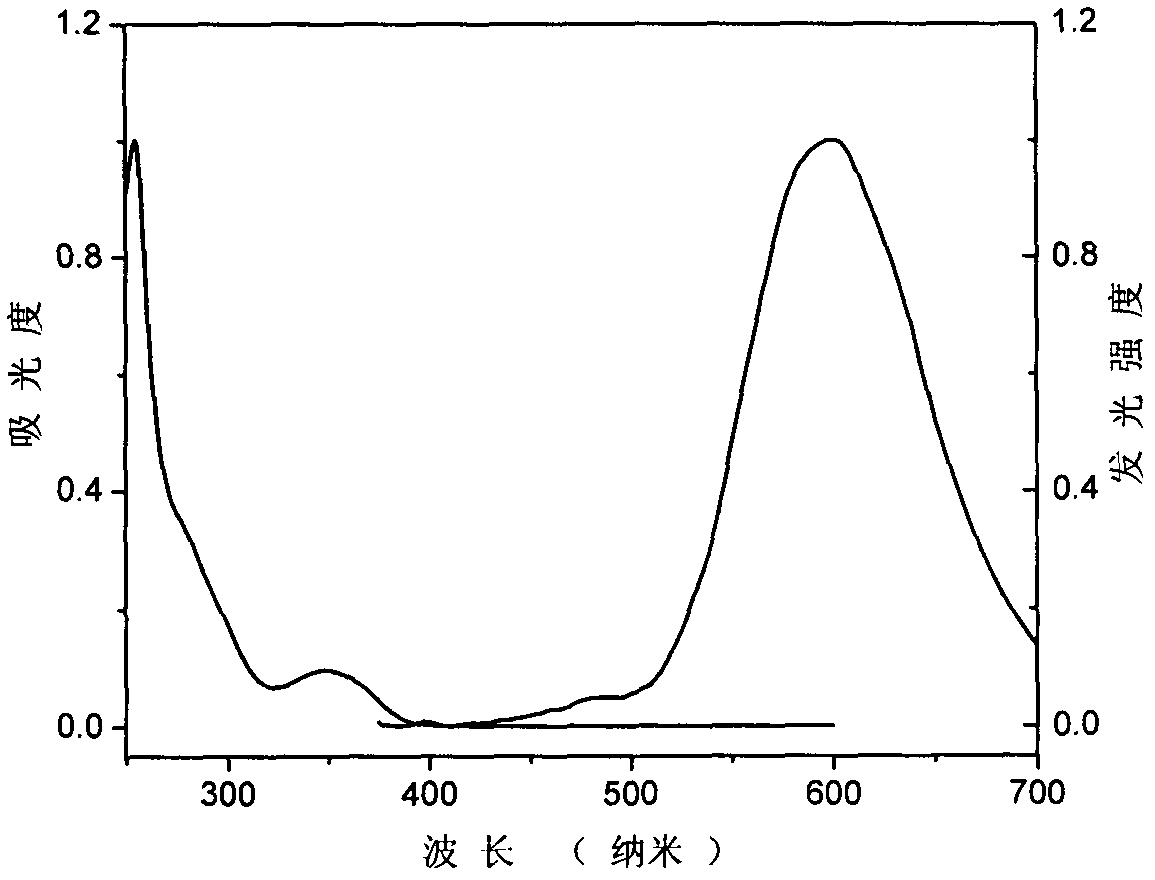
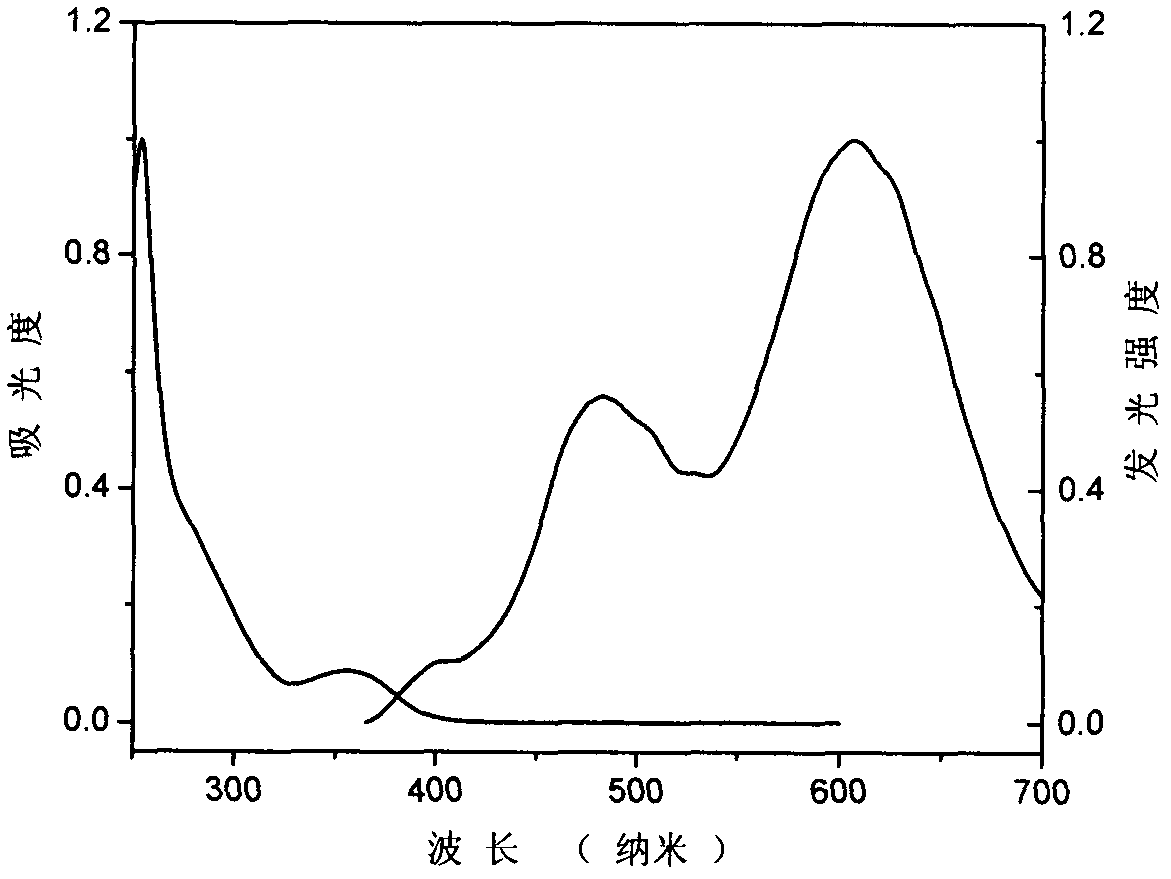
[0044] In a 100ml three-necked bottle, add N in sequence 9 , N 10 -Diphenylphenanthrene-9,10-diamine (4.0g, 11.1mmol), iodobenzene (3.4g, 16.6mmol), potassium carbonate (3.1g, 22.2mmol), cuprous chloride (0.7g, 17.1mmol ), trichlorobenzene 15ml, turn on stirring and heating, react at 150°C for 1 hour, then continue to heat up to 210°C for reaction, track the reaction until the raw materials are completely reacted. After the reaction, the trichlorobenzene was distilled off under reduced pressure to obtain a brown-black viscous object. Petroleum ether: dichloromethane = 20:1: passed through the column (silica gel column) to obtain 1.0 g of white solid (Compound Ia), with a yield of 21%. The ultraviolet-visible absorption spectrum figure and fluorescence spectrum figure of compound I a are shown in figure 1 , the cyclic voltammetry curve of compound I a is shown in Figure 4 , the ...
Embodiment 2
[0047] Synthesis of 3-methyl-1-p-toluene-6-benzene-benzo[b]-dibenzo[f,h]quinoxaline (compound I b)
[0048]
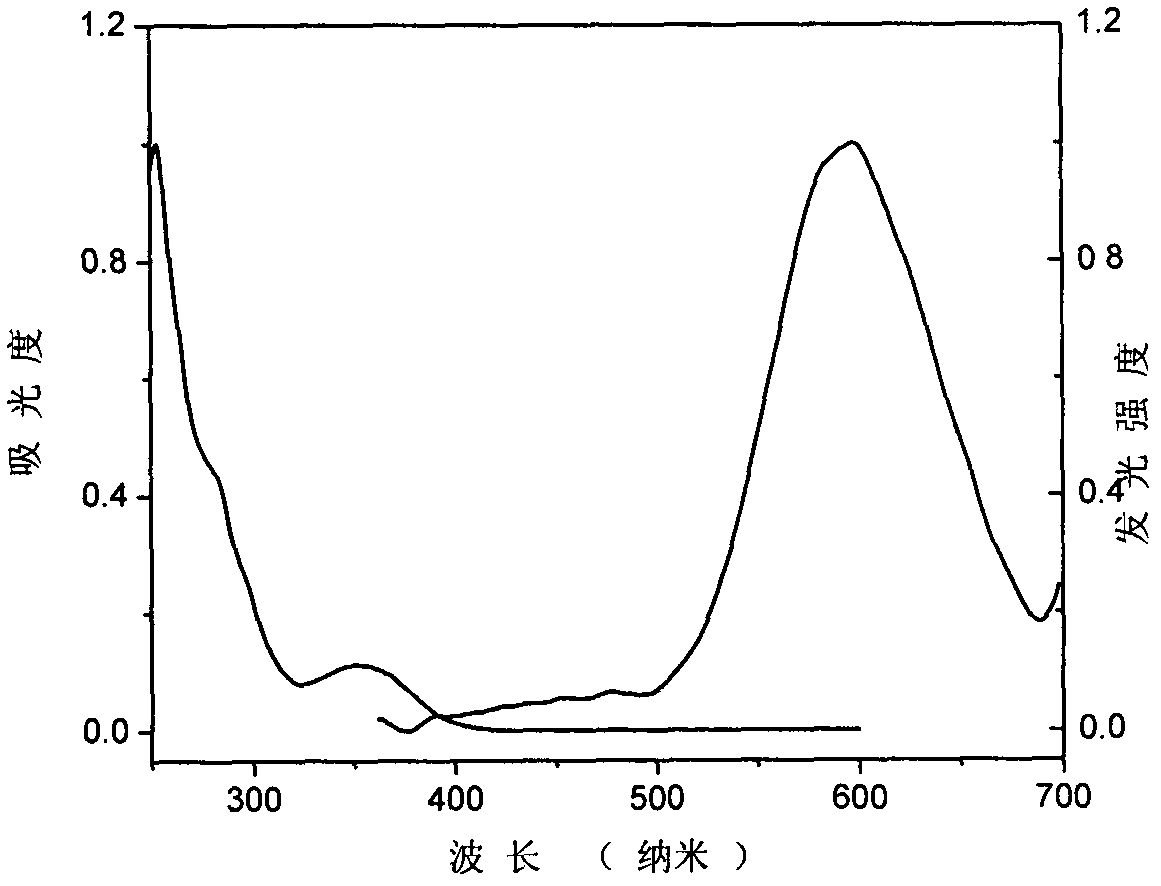
[0049] In a 100ml three-necked bottle, add N in sequence 9 , N 10 -bis(4-methylbenzene)-9,10-diaminophenanthrene (4.0g, 10.3mmol), iodobenzene (3.2g, 15.5mmol), potassium carbonate (2.8g, 20.6mmol), cuprous chloride ( 0.7g, 17.1mmol), trichlorobenzene 15ml, start stirring and heating, react at 150°C for 1 hour, then continue to heat up to reflux reaction, track the reaction until the raw materials are completely reacted. After the reaction, the trichlorobenzene was distilled off under reduced pressure to obtain a brown-black viscous object. Petroleum ether: dichloromethane = 20:1: passed through the column (silica gel column) to obtain 0.8 g of white solid (Compound Ib), with a yield of 18%.
[0050] 1 H NMR (400MHz, CDCl 3 ): δ=8.72(d, J=8.3Hz, 2H), 8.10(dd, J=15.2, 7.9Hz, 2H), 7.74-7.40(m, 6H), 7.12(d, J=9.0Hz, 1H) , 7.06-6.98(m, 2H), 6.93(d, J=9.0Hz, 4H), 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com