Ethylenedioxothiophene-and-naphthalene-tetracarboxylic-acid-bisimide-structural-unit-based low band gap polymers, and preparation method and application method thereof
A technology of tetracarboxylic diimide and ethylenedioxythiophene, which is applied in the fields of electrical components, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc., and can solve the problems of low polymer yield and low polymer molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
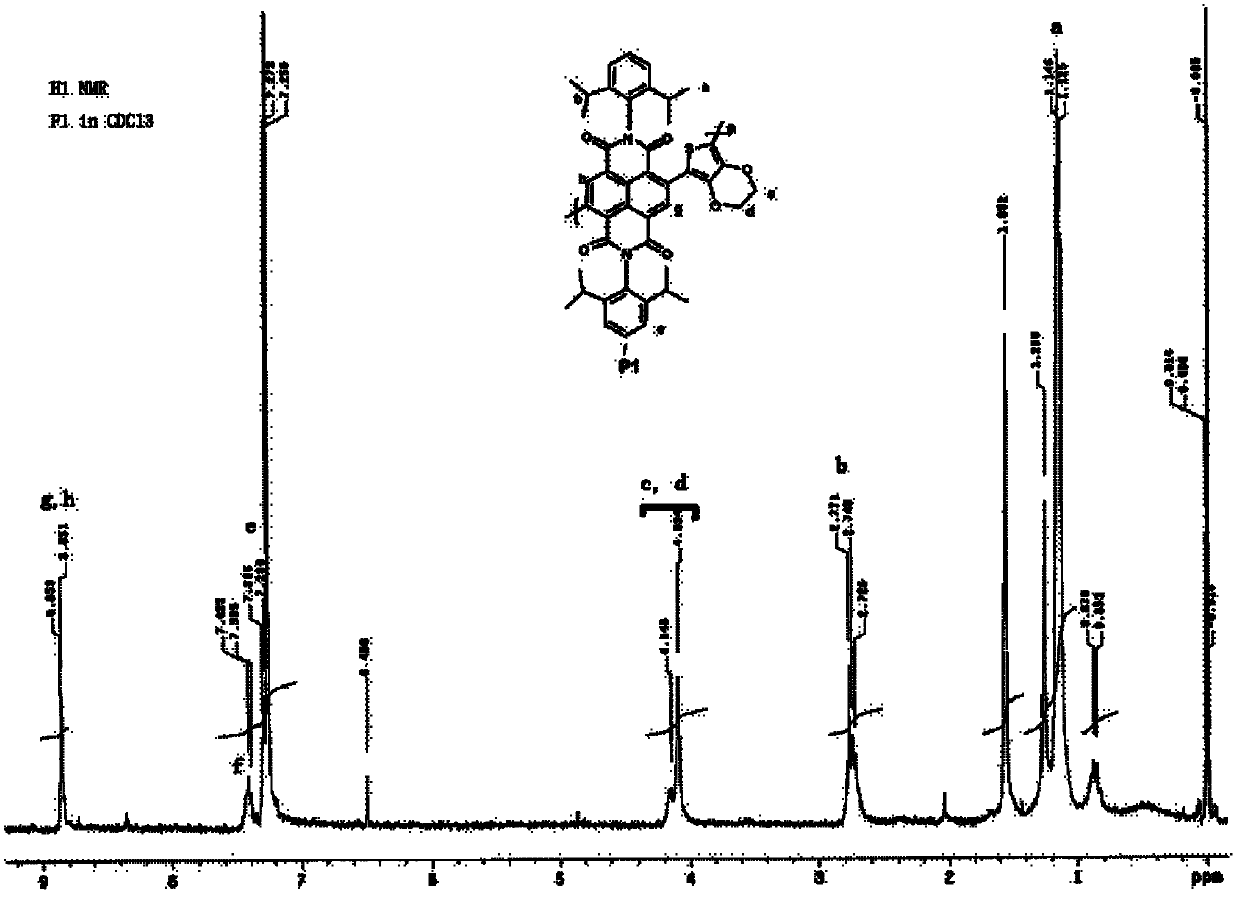
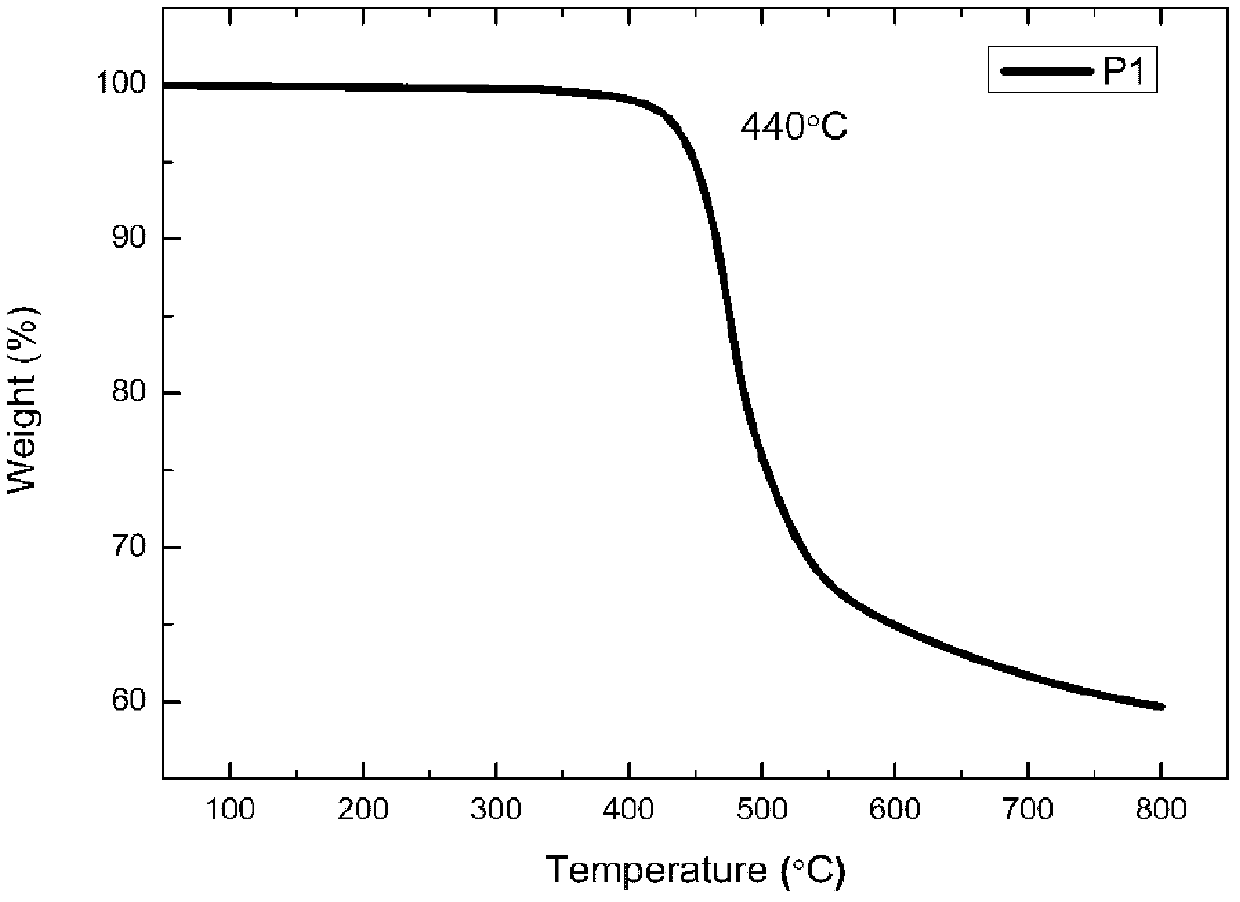
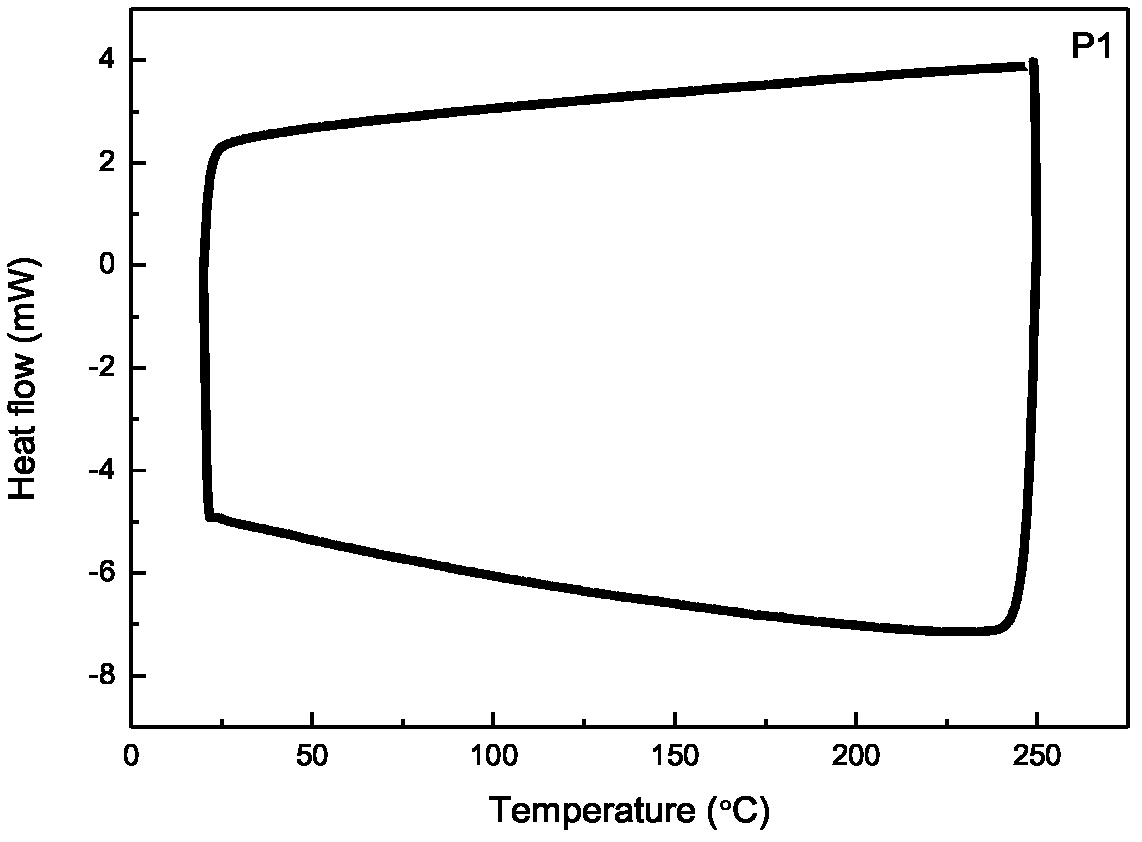
[0027] Embodiment 1 prepares alternating copolymer P1
[0028] 1) Preparation of alternating copolymer P1
[0029] Monomer N,N'-bis-(2',6'-diisopropylphenyl)-2,6-dibromonaphthalene-1,4,5,8-tetracarboxylic acid diimide (1) and Monomer 2,5-bis(tributyltin)-3,4-ethylenedioxythiophene (2) is polymerized by Stille coupling.
[0030]
[0031] Specifically: first place the monomers (1) and (2) and the catalyst tris(dibenzylideneacetone) dipalladium (0), triphenylarsenic and cuprous iodide in a long-necked sealed reaction with a branch In the bottle, use a double-row tube to evacuate, nitrogen, and then evacuate, repeat this three times to completely remove the oxygen in the system, then add the newly distilled tetrahydrofuran solvent with a syringe, and then blow nitrogen with positive pressure to remove oxygen for 20 minutes, then completely seal the reaction bottle with a sealing tape, heat it to 80°C with an oil bath for reaction, and keep it for 72 hours, and then undergo po...
Embodiment 2
[0047] Embodiment 2 prepares random copolymer P2
[0048] 1) Preparation of random copolymer P2:
[0049] By adding 2,5-dibromo-3,4-ethylenedioxythiophene (3) monomer, and 2,5-di(tributyltin)-3,4-ethylenedioxythiophene (2) and N, N'-bis-(2',6'-diisopropylphenyl)-2,6-dibromonaphthalene-1,4,5,8-tetracarboxylic acid diimide (1) monomer, participating together Random copolymer P2 was prepared by Stille coupling polymerization.
[0050]
[0051] The purpose of the design of this embodiment is to increase the component of the electron donor unit 3,4-ethylenedioxythiophene in the copolymer product, so as to achieve the purpose of further red-shifting its absorption spectrum and having a relatively broader absorption. The ultraviolet spectrum shows that the expected effect has been obtained. Wherein, 3,4-ethylenedioxythiophene was brominated with N-bromosuccinimide in a mixed solution of chloroform and glacial acetic acid, and purified by column chromatography and recrystallizat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com