Synthetic method of calcium fluoride
A synthetic method and calcium fluoride technology, applied in the direction of calcium/strontium/barium fluoride, calcium/strontium/barium halide, etc., can solve the problems of fluorite price rise, normal production, and close physical properties, etc., to achieve good Social and economic benefits, solving effects that are difficult to use efficiently
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
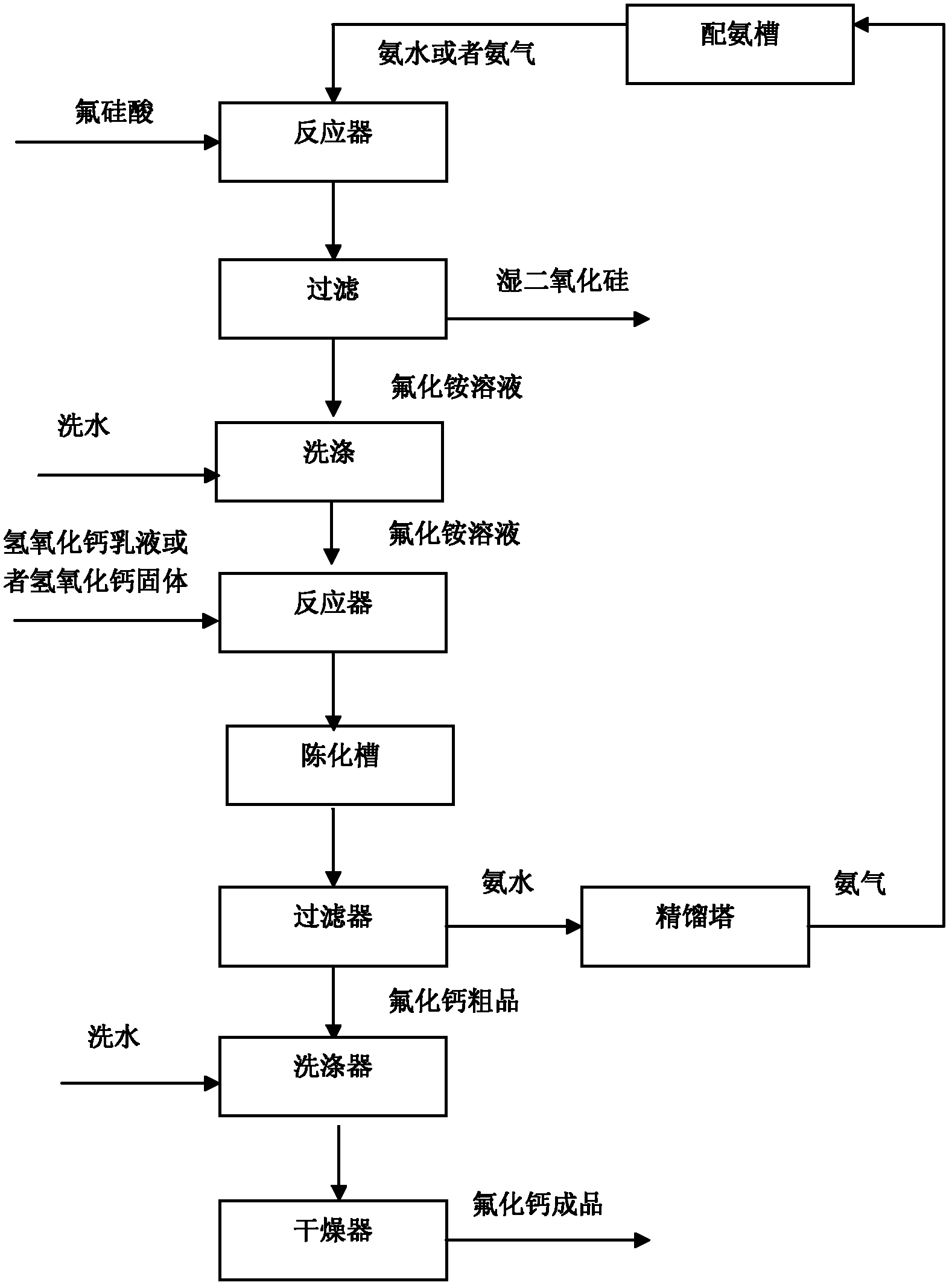
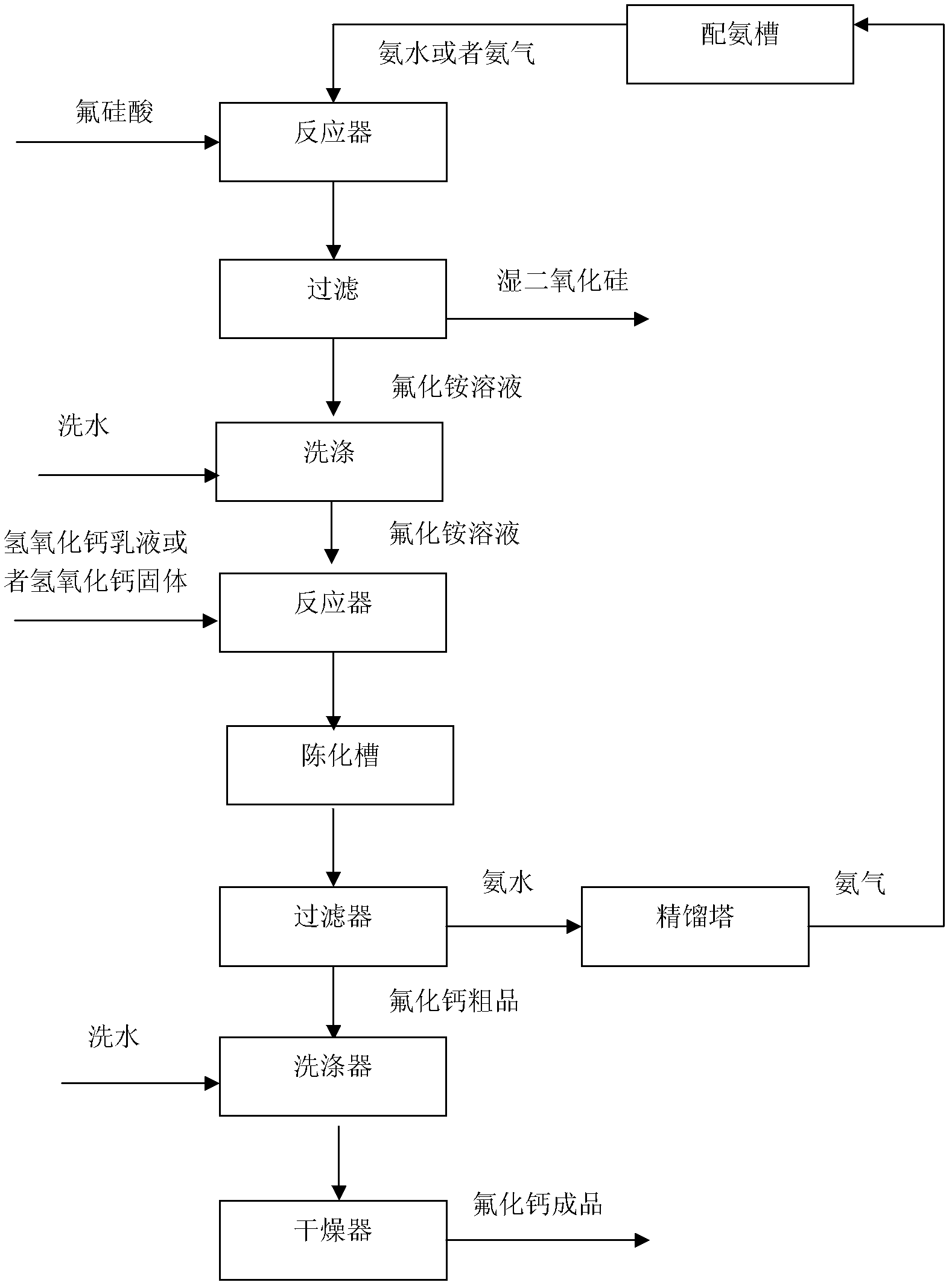
[0026] After injecting 3000g of fluosilicic acid with a concentration of 5% into the pressurized stirring reactor, feed 2550g of ammonia water with a concentration of 5%, and start stirring while adding ammonia water. The stirring speed is 500rpm, and the reaction pressure is controlled to 0.3MPa. The temperature was 140°C, and after 180 minutes of reaction, the reaction materials were filtered and washed to obtain 5472g of ammonium fluoride solution with a concentration of 4.07%. The filter cake was 150g of wet silica with a water content of 60% and 80g of washing water. Add the ammonium fluoride solution into the ultrasonic reactor, turn on the ultrasonic wave with a frequency of 40KHz, start stirring at a stirring speed of 100rpm, add 220g of calcium hydroxide into the ultrasonic reactor at a uniform speed, the feeding time is 60min, and the reaction temperature is 80°C. The reaction time is 120min. After the reaction is completed, the material is poured into the aging tank,...
Embodiment 2
[0028] After injecting 750g of fluorosilicic acid with a concentration of 20% into the pressurized stirring reactor, feed 638g of ammonia water with a concentration of 20%, and start stirring while adding ammonia water. The stirring speed is 500rpm, and the reaction pressure is controlled at 0.2MPa. The temperature was 80°C, and after reacting for 90 minutes, the reaction materials were filtered and washed to obtain 1320 g of ammonium fluoride solution with a concentration of 16.65%. The filter cake was 148 g of wet silica with a water content of 60%, and the amount of washing water was 80 g. Add the ammonium fluoride solution into the reactor, start stirring, and the stirring speed is 100rpm, add 730g of calcium hydroxide emulsion with a concentration of 30% into the reactor at a uniform speed, the feeding time is 40min, the reaction temperature is 60°C, and the reaction time is 60min, after the reaction is completed, the material is lowered from 50°C to 20°C, the cooling rate...
Embodiment 3
[0030] After injecting 375g of fluorosilicic acid with a concentration of 40% into the pressurized stirring reactor, feed 425g of ammonia water with a concentration of 30%, and start stirring while adding ammonia water. The stirring speed is 100rpm, and the reaction is carried out under normal pressure and normal temperature. After reacting for 30 minutes, the reaction materials were filtered and washed to obtain 1095 g of ammonium fluoride solution with a concentration of 20.07%, and the filter cake was 138 g of wet silica with a water content of 58%, and the amount of washing water was 80 g. Add the ammonium fluoride solution into the ultrasonic reactor, turn on the ultrasonic wave, the frequency is 20KHz, start stirring, the stirring speed is 200rpm, and add 1075g of calcium hydroxide emulsion with a concentration of 20% into the ultrasonic reactor at a uniform speed, and the feeding time is 20min. The temperature is 20°C, and the reaction time is 30 minutes. After the react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com