Method for preparing alkali zinc chloride nano-powder in hexagonal flake structures
A nano-powder, flake-like technology, applied in the field of nano-materials, can solve the problems of high risk, low yield, cumbersome steps, etc., and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Choose anhydrous zinc chloride (ZnCl 2 ) as a zinc salt reagent (0.2mol / l), hexamethylenetetramine as a hydroxyl reagent (0.2mol / l), the molar ratio of the two is 1:1, and the volume ratio is 20 milliliters of water-ethanol solution of 1:1 As a solvent, prepare the precursor solution for the ZHC reaction. Dissolve anhydrous zinc chloride in water first, then add hexamethylenetetramine under stirring condition, adjust the pH value of the solution to 6.0 with dilute hydrochloric acid, add ethanol, and obtain the ZHC precursor solution after static defoaming.
[0022] After the precursor solution was prepared, it was placed in an oven and heated from room temperature to 70°C for 1 hour.
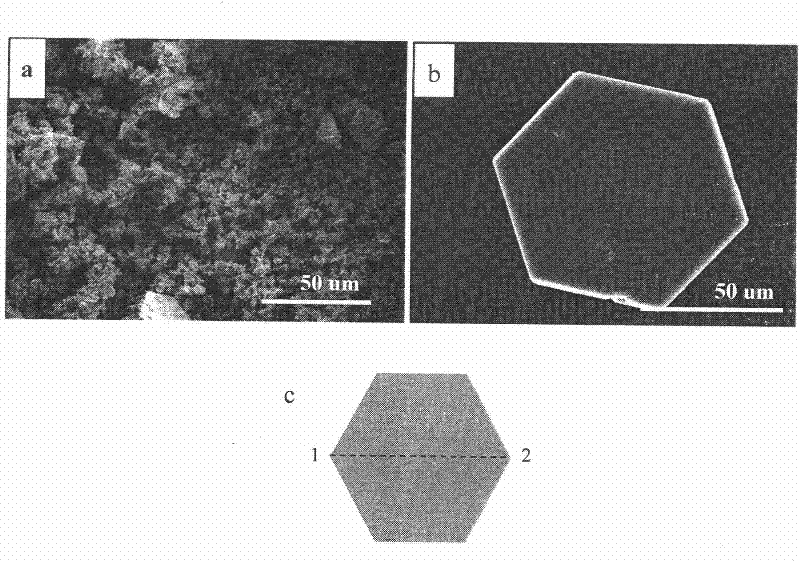
[0023] Use a centrifuge to collect the white precipitate at the bottom of the container, wash it with water and ethanol three times, and dry it in an oven at 105°C to obtain ZHC nanoflake powder.
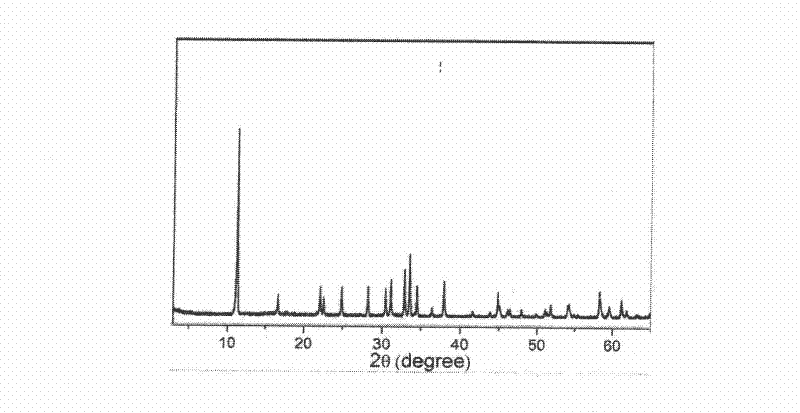
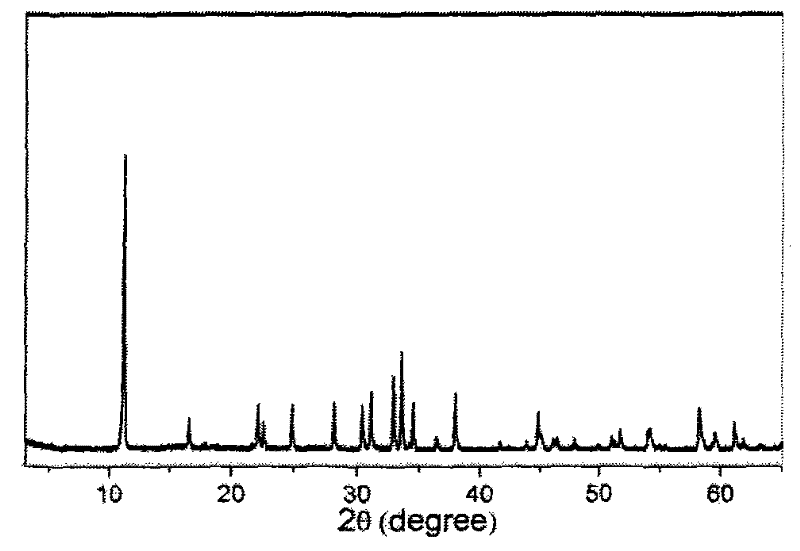
[0024] From attached figure 1 The provided X-ray diffraction pattern shows that the obta...
Embodiment 2
[0029] Zinc chloride hydrate (ZnCl 2 .4H 2 O) is a zinc salt reagent (0.1mol / l), hexamethylenetetramine is as a hydroxyl reagent (0.2mol / l), and the molar ratio of the two is 1: 2, and the volume ratio is 20 milliliters of water-ethyl alcohol of 3: 1 The diol solution is used as a solvent to prepare the precursor solution for the ZHC reaction. Dissolve zinc chloride hydrate in water first, then add hexamethylenetetramine under stirring condition, adjust the pH value of the solution to 6.0 with dilute hydrochloric acid, add ethanol, and obtain the ZHC precursor solution after static defoaming.
[0030] After the precursor solution is prepared, it is placed in a water bath at 70° C., and the reaction time is 4 hours. Use a centrifuge to collect the white precipitate at the bottom of the container, wash it with water and ethanol three times, and dry it in an oven at 105°C to obtain ZHC nanoflake powder. The product morphology is close to Example 1.
Embodiment 3
[0032] Choose anhydrous zinc chloride (ZnCl 2 ) is zinc salt reagent (0.05mol / l), urea is as hydroxyl reagent (0.2mol / l), and both molar ratio is 1: 2, and volume ratio is 20 milliliters of water-ethylene glycol solutions of 1: 3 as solvent, Prepare the precursor solution for the ZHC reaction. First dissolve zinc chloride hydrate in water, then add hexamethylenetetramine under agitation, adjust the pH value of the solution to 4.0 with dilute hydrochloric acid, add an appropriate amount of ethylene glycol, and get the ZHC precursor solution after static defoaming .
[0033] After the precursor solution is prepared, it is placed in a water bath at 95°C, and the reaction time is 24 hours. Use a centrifuge to collect the white precipitate at the bottom of the container, wash it with water and ethanol three times, and dry it in an oven at 105°C to obtain ZHC nanoflake powder. The morphology of the obtained ZHC nanosheets was similar to Example 1, but the thickness was increased ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com