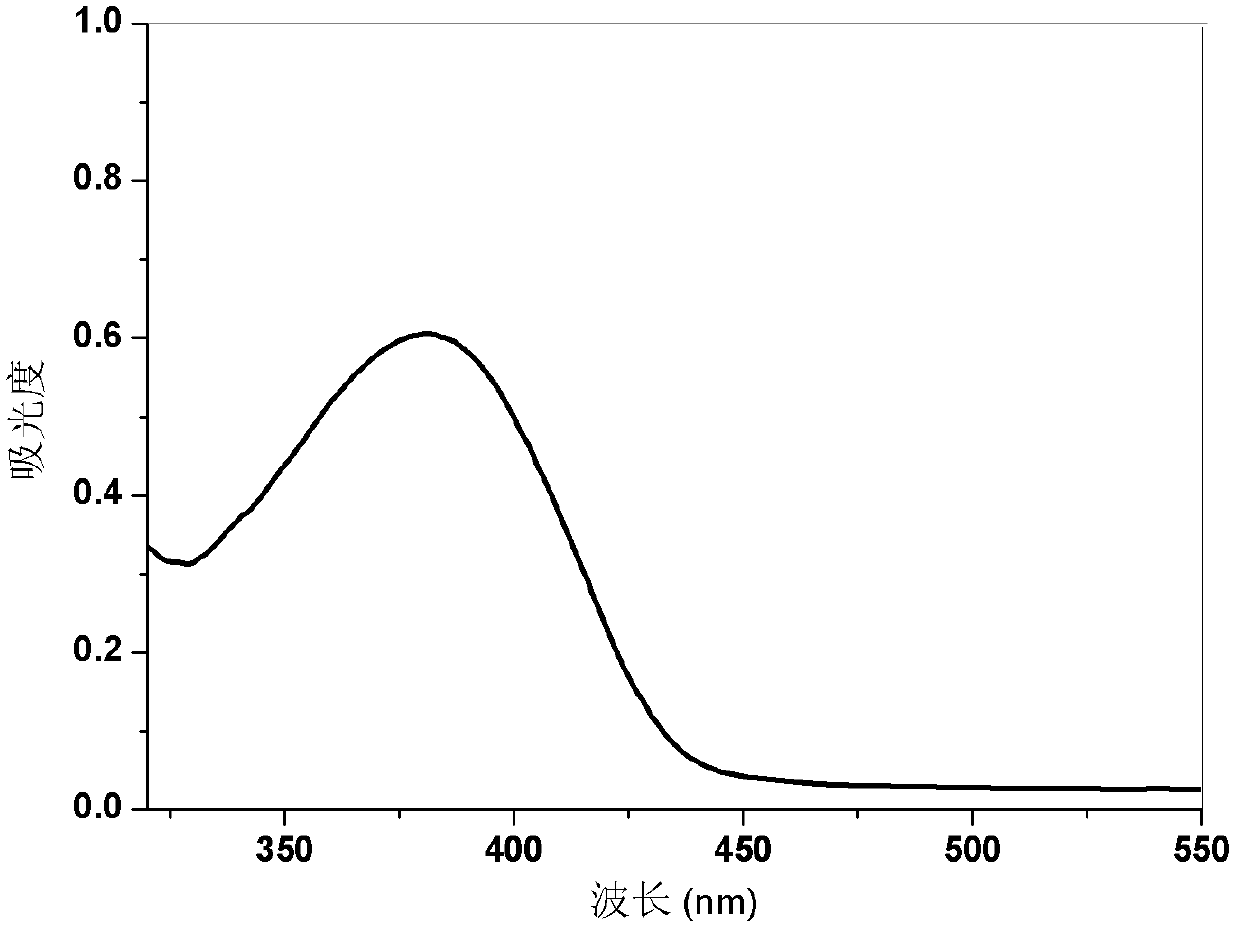
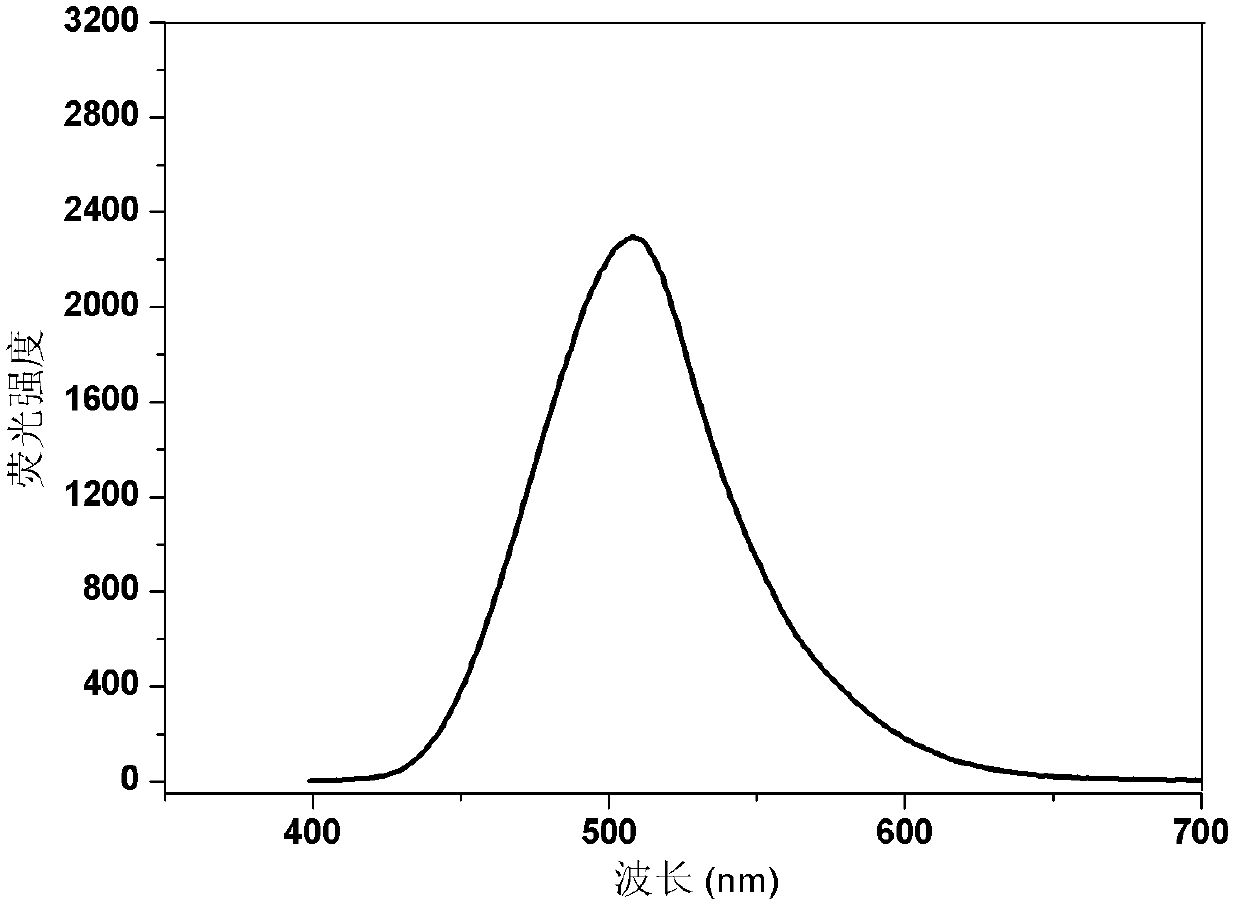
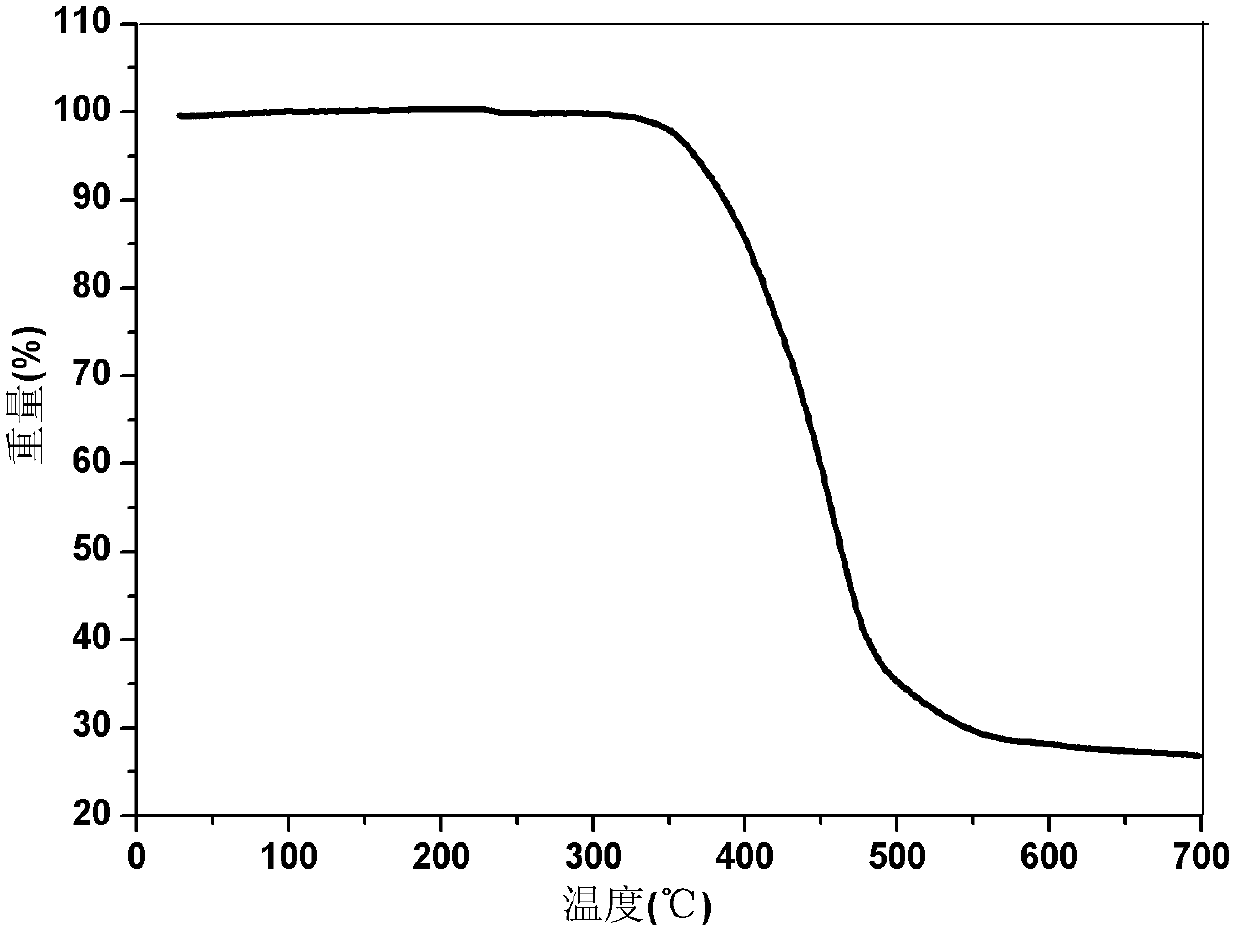
Dibenzothiophene chalcone containing coumarin skeleton and synthesis method and application thereof
A technology of dibenzothiophene and coumarin skeleton, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of unreported photophysical and photochemical properties, and achieve good ultraviolet absorption and fluorescence emission performance. , The synthesis and separation methods are simple, and the stability is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of 3-(3-Dibenzothiophene-2-acryl)benzopyran-2-one
[0054] The synthesis proceeds in three steps:
[0055] (1) Synthesis of 3-dibenzothiophenealdehyde
[0056] Dibenzothiophene (1.1 g, 6 mmol) and 20 mL of anhydrous Cl 2 CH 2 Add it to a three-necked flask equipped with a constant pressure funnel, stir and dissolve in an ice-water bath; take 1,1-dichloromethyl methyl ether (1 mL, 12 mmol), use 10 mL of anhydrous Cl 2 CH 2 Dissolve, add to dibenzothiophene; after 10 minutes, take titanium tetrachloride (1.4 mL, 13 mmol) and dissolve it in 20 mL of anhydrous Cl 2 CH 2 , slowly added dropwise to the dibenzothiophene mixed solution, the dropwise addition was completed in about 20 minutes, and the reaction was continued at 25 ° C for 4 hours; the solution was poured into ice water (more ice), vigorously stirred for 1 hour, and the solution was divided into two layers , use anhydrous Cl for the aqueous phase 2 CH 2 Extraction, combine the organic phases, then ...
Embodiment 2
[0066] Synthesis of 3-(3-dibenzothiophene-2-acryloyl)-5,6-benzobenzopyran-2-one
[0067] The synthesis is carried out in three steps:
[0068] (1) Synthesis of 3-dibenzothiophenealdehyde
[0069] With embodiment 1 step (1);
[0070] (2) Synthesis of 3-acetyl-5,6-benzocoumarin
[0071] 2-Hydroxy-1-naphthaldehyde (1.72g, 10mmol) was mixed with ethyl acetoacetate (1.3g, 10mmol) and added to the three-necked flask, then dissolved in 15mL of absolute ethanol solvent, stirred in an ice-water bath, and then added 0.5mL (5mmol) of hexahydropyridine as a The catalyst was stirred in an ice-water bath for 6 hours, filtered with suction, the crude product was washed with deionized water and ethanol, and then recrystallized with absolute ethanol and acetone to obtain a light yellow solid product with a yield of about 76%.
[0072] The structural characterization data of the product is: m.p.189~190℃; 1 H NMR (400MHz, CDCl 3 / TMS)δ: 2.81(s, 3H), 7.51(d, J=9.0Hz, 1H), 7.64(t, J=8.0Hz, ...
Embodiment 3
[0079] Synthesis of 3-(3-dibenzothiophene-2-acryloyl)-7-diethylaminobenzopyran-2-one
[0080] The synthesis is carried out in three steps:
[0081] (1) Synthesis of 3-dibenzothiophenealdehyde
[0082] With embodiment 1 step (1);
[0083] (2) Synthesis of 3-acetyl-7-diethylaminocoumarin
[0084] The 4-N,N-% ethylaminosalicylaldehyde (1.0g, 6.1mmol) and ethyl acetoacetate (0.84g, 6.1mmol) were mixed into the three-necked flask, then dissolved in 15mL absolute ethanol solvent, stirred in an ice-water bath, and then added 0.3mL (3mmol) hexahydrogen Pyridine was used as a catalyst, the reaction was stirred in an ice-water bath for 8 hours, filtered with suction, and the crude product was washed with deionized water and ethanol, and then recrystallized with absolute ethanol and acetonitrile to obtain a bright yellow solid product with a yield of about 69%.
[0085] The structural characterization data of the product are: m.p.151~153℃; 1 H NMR (400MHz, CDCl 3 / TMS)δ: 8.49(s, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com