Magnetically-separable noble metal catalyst and preparation method thereof
A noble metal catalyst and magnetic separation technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino hydroxyl compounds, etc., can solve the problems of unsatisfactory separation efficiency, difficult separation of catalysts, and long time consumption, etc., and achieve excellent catalytic activity , Good recyclability, easy to separate and recycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Preparation of magnetic core with core-shell structure
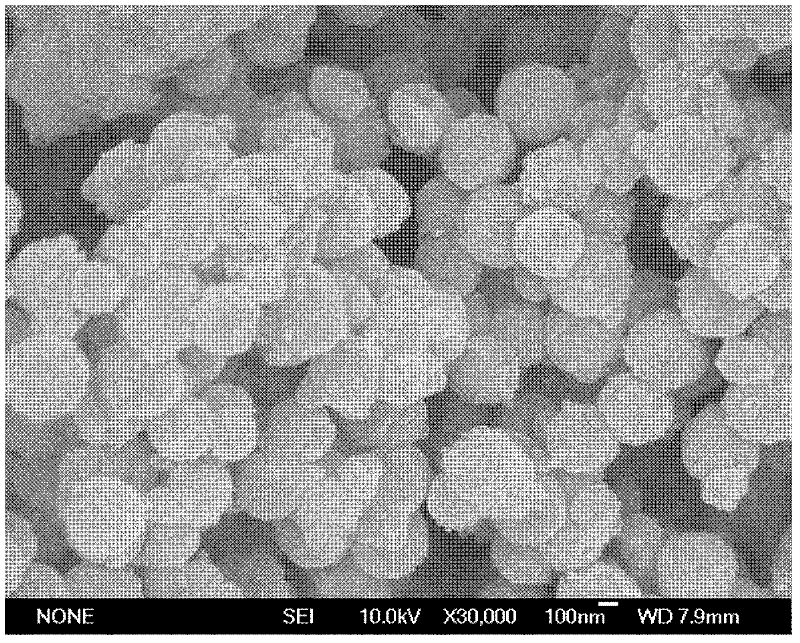
[0016] First, ferric oxide microspheres were prepared. 2.7 g FeCl 3 ·6H 2 O and 7.2 g of sodium acetate were added to 100 mL of ethylene glycol solution, stirred magnetically until a uniform yellow clear solution was formed, then transferred to a polytetrafluoroethylene-lined high-pressure hydrothermal kettle, and reacted at 200 ° C for 8 h. The obtained iron tetroxide magnetic particles were repeatedly washed with water and ethanol, dried under vacuum at 50° C. and then used for future use. Before loading the silica shell layer on the surface of ferric oxide, 0.1 g of ferric oxide was ultrasonically treated with 15 mL of 2M HCl solution for 5 min. Magnetically separated, washed, and then added to a mixed solution of 400mL ethanol, 100mL ultrapure water and 15mL concentrated ammonia water. After mechanical stirring for 15 minutes, 3.5mL tetraethyl orthosilicate was added dropwise, and mechanical stir...
Embodiment 2
[0017] Embodiment 2: Preparation of gold nanoparticles
[0018] 250mL HAuCl 4 ·3H 2 O aqueous solution (1 mM) and 25 mL aqueous sodium citrate solution (38.3 mM) were both heated to boiling with magnetic stirring. Then the boiling sodium citrate solution was quickly added to the gold precursor solution, the mixed solution was stirred for 10 minutes, the heat source was removed, and the solution was stirred for 15 minutes to obtain a purple-red solution containing gold nanoparticles. After cooling, refrigerate and store for later use.
Embodiment 3
[0019] Example 3: Loading of gold nanoparticles on magnetic microspheres
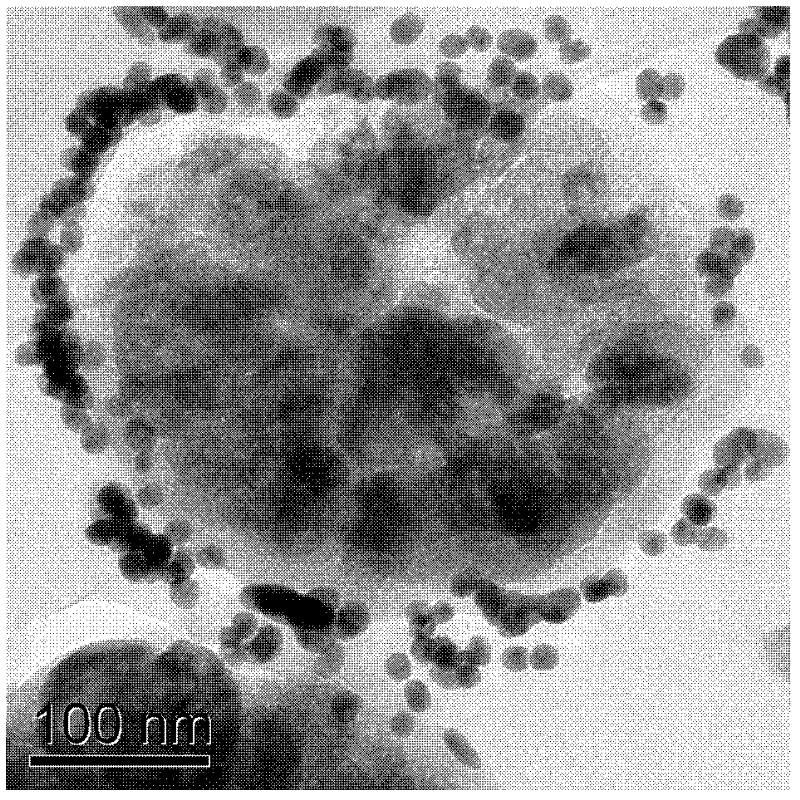
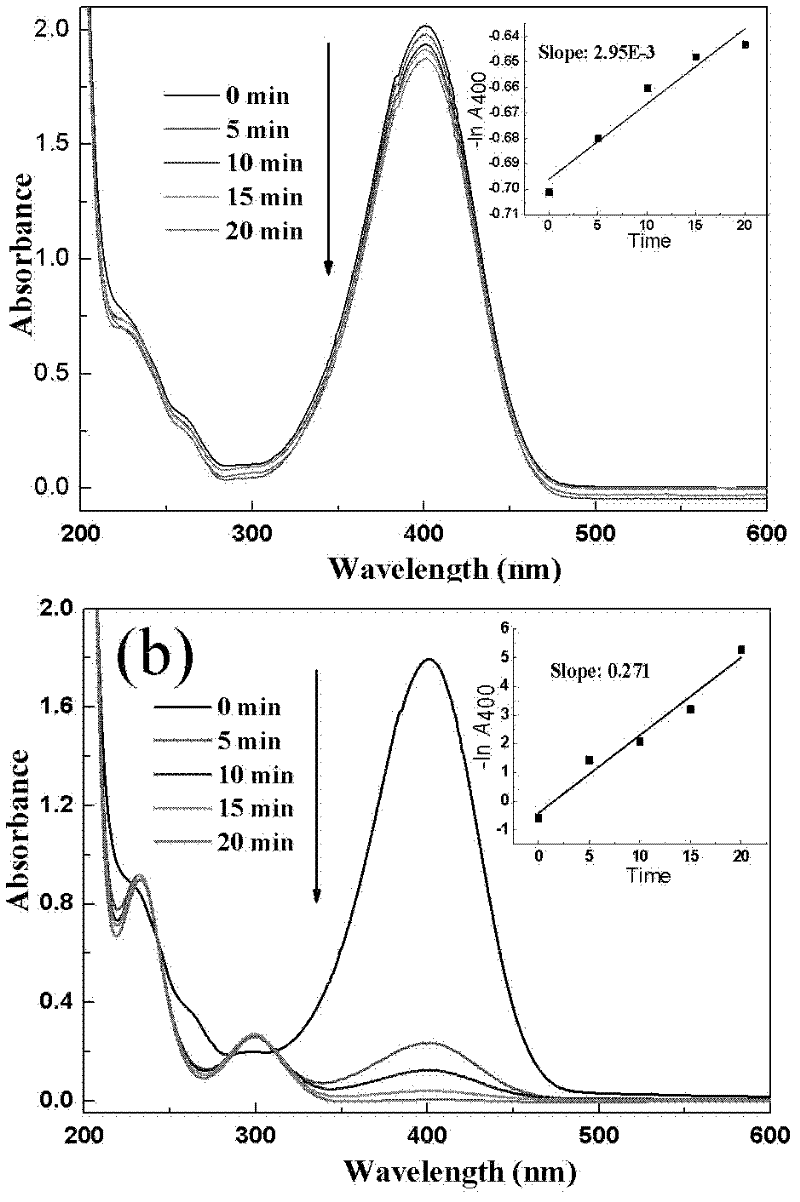
[0020] The loading of gold nanoparticles on magnetic microspheres is carried out by the layer-by-layer self-assembly method according to the following steps: (i) 0.25g of magnetic microsphere carrier with core-shell structure is first added to 10mL concentration of 10mg / mL polyethylene diamine ( PEI) aqueous solution, stirred for 30 minutes, and a layer of PEI polycations was adsorbed on the surface of the microspheres. Then magnetic separation, repeated washing to remove excess PEI. The PEI solution contained 0.5M NaCl as a supporting electrolyte, and the pH of the solution was adjusted to 8.5. (ii) The magnetic microspheres adsorbed by PEI were added to 10mL of the pre-synthesized Au solution, stirred for 30min, and a layer of gold nanoparticles was adsorbed on the surface of the PEI polycation, and the above separation and washing process was repeated to obtain a PEI / Au bilayer magnetic microspher...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com