Method for preparing tebipenem
A molar ratio and compound technology, applied in the field of chemistry or medicinal chemistry, can solve the problems of increased operation difficulty, increased impurities, reduced yield and quality, etc., and achieve the effect of simple post-treatment operation, reduced impurities, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
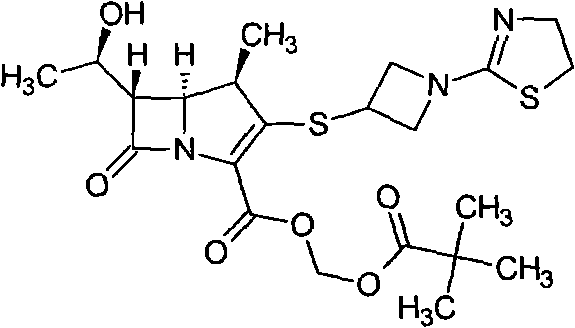
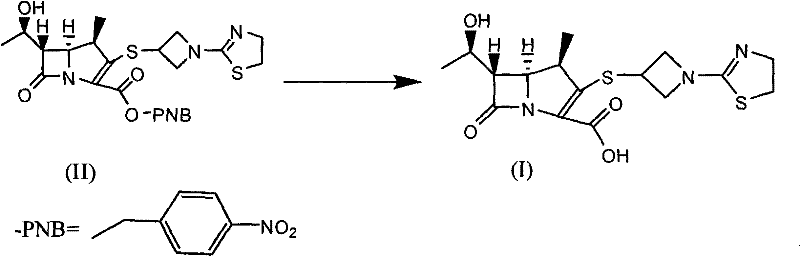
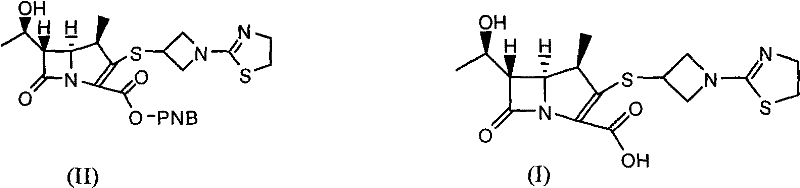
[0034] (4R, 5S, 6S)-3-[[1-(4,5-dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-[(1R)-1- Preparation of hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (compound of formula (I))
[0035] (4R, 5S, 6S)-3-[[1-(4,5-dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-[(1R)-1- Hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid p-nitrobenzyl ester (formula (II) compound) 23.30g ( 45mmol) was dissolved in 275ml of n-butanol, 340ml of water and 2,6-lutidine 14.44g (135mmol), added 10% palladium / carbon 3.50g, transferred to a 2L reactor, and the reaction solution was at a pressure of 1.1MPa Under 26°C, the reaction time is about 3 hours. Palladium carbon was removed by filtration, the water layer was separated, and 915 ml of acetone was slowly dropped into the water layer for crystallization while cooling and stirring in an ice bath. The off-white product was obtained by suction filtration, and dried overnight under reduced pressure to obtain the...
Embodiment 2
[0037] (4R, 5S, 6S)-3-[[1-(4,5-dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-[(1R)-1- Preparation of hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (compound of formula (I))
[0038] (4R, 5S, 6S)-3-[[1-(4,5-dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-[(1R)-1- Hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid p-nitrobenzyl ester (formula (II) compound) 23.30g ( 45mmol) was dissolved in 450ml tetrahydrofuran, 300ml water, 14.44g (135mmol) of 2,4-lutidine, 2.33g of 10% palladium / carbon was added, and transferred to a 2L reaction kettle. The reaction time was 3 hours at °C. Palladium-carbon was removed by filtration, the water layer was separated, and 900ml of acetone was slowly dropped into the water layer for crystallization while cooling and stirring in an ice bath. The off-white product was obtained by suction filtration, and dried overnight under reduced pressure to obtain the off-white product (4R, 5S, 6S)-3-[[1-(4,5-dihy...
Embodiment 3
[0040] (4R, 5S, 6S)-3-[[1-(4,5-dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-[(1R)-1- Preparation of hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (compound of formula (I))
[0041] (4R, 5S, 6S)-3-[[1-(4,5-dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-[(1R)-1- Hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid p-nitrobenzyl ester (formula (II) compound) 23.30g ( 45mmol) was dissolved in 275ml n-butanol, 340ml water and 2,6-lutidine 24.09g (225mmol), counted in 10% palladium hydroxide palladium / carbon 3.50g, transferred in the 2L reactor, and the reaction solution was The pressure was 0.7 MPa and the temperature was 50° C. for 3 hours. Palladium carbon was removed by filtration, the water layer was separated, and 900 ml of acetone was slowly dropped into the water layer for crystallization while cooling and stirring in an ice bath. The off-white product was obtained by suction filtration, and dried overnight under reduced pres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com