EB (Epstein-Barr) virus Zta IgA (Immunoglobulin A) antibody colloidal gold detection kit and preparation method thereof
A detection kit and EB virus technology, applied in the field of medicine and biology, can solve the problems of complex operation and long detection time of ELISA method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
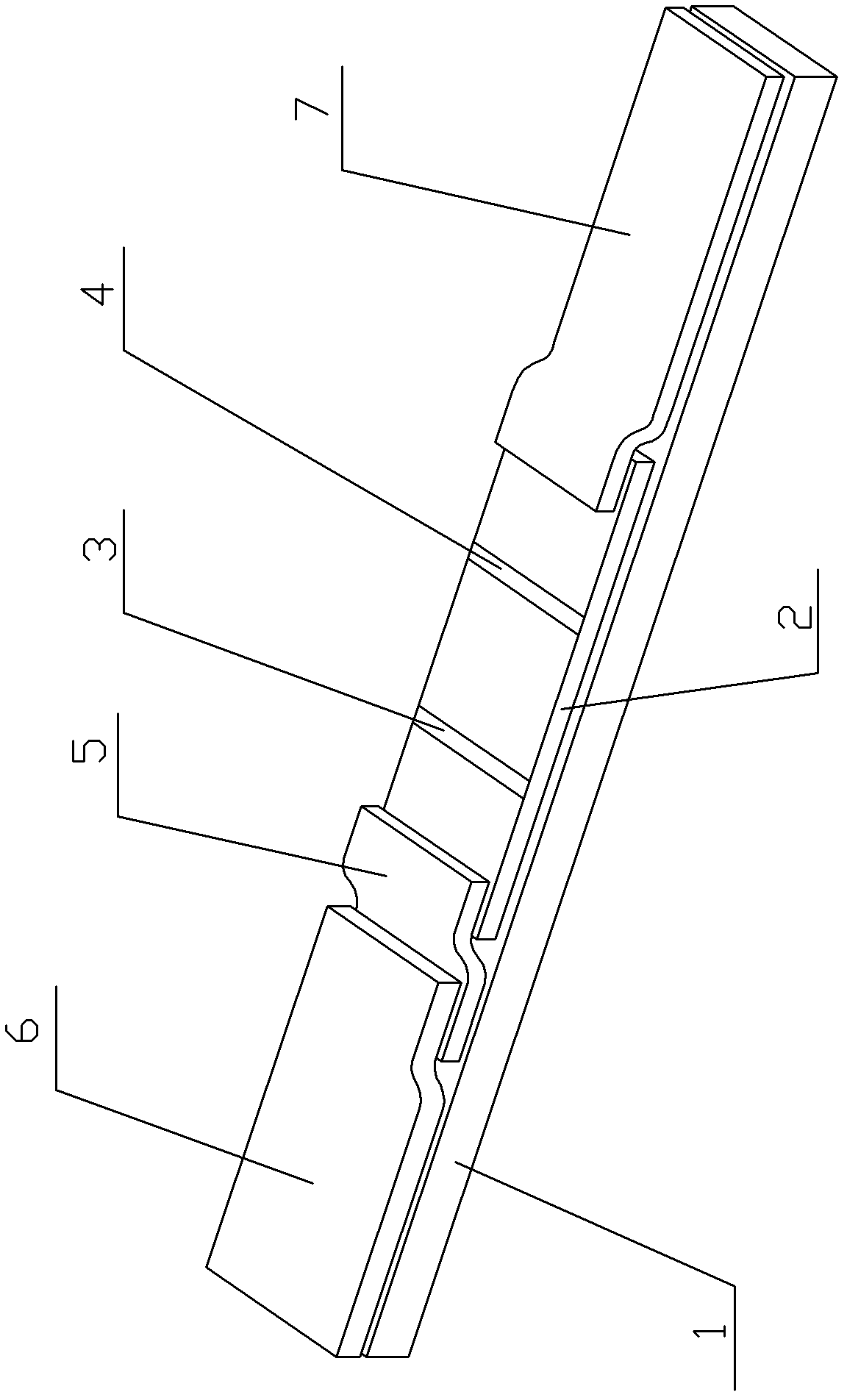
[0109] The base plate 1 is a PVC plate, and the reaction membrane 2 is an NC film. Embodiment 1: the preparation of kit
[0110] 1. Reagent preparation
[0111] (1) Preparation of 0.05M PBS buffer solution (according to 100ml volume):
[0112] Accurately weigh Na 2 HPO 4 12H 2 O 1.450g, NaH 2 PO 4 2H 2 O 0.148g, NaCl 0.850g, add 80.00mL of purified water, stir to fully dissolve, then dilute to 100.00mL with purified water, mix well, store at 2~8℃ for later use, valid for 14 days.
[0113] (2) Preparation of 1% chloroauric acid solution (according to 100ml amount):
[0114] Weigh 1g of chloroauric acid, add 80ml of purified water to dissolve it, add purified water to make up to 100ml, mix well, wrap it in aluminum foil, and store it at 2-8°C for later use, with a validity period of 12 months.
[0115] (3) Preparation of 0.1M potassium carbonate solution (according to 20ml volume):
[0116] Measure 16ml of purified water into the reagent bottle, and accurately weigh 0....
Embodiment 2
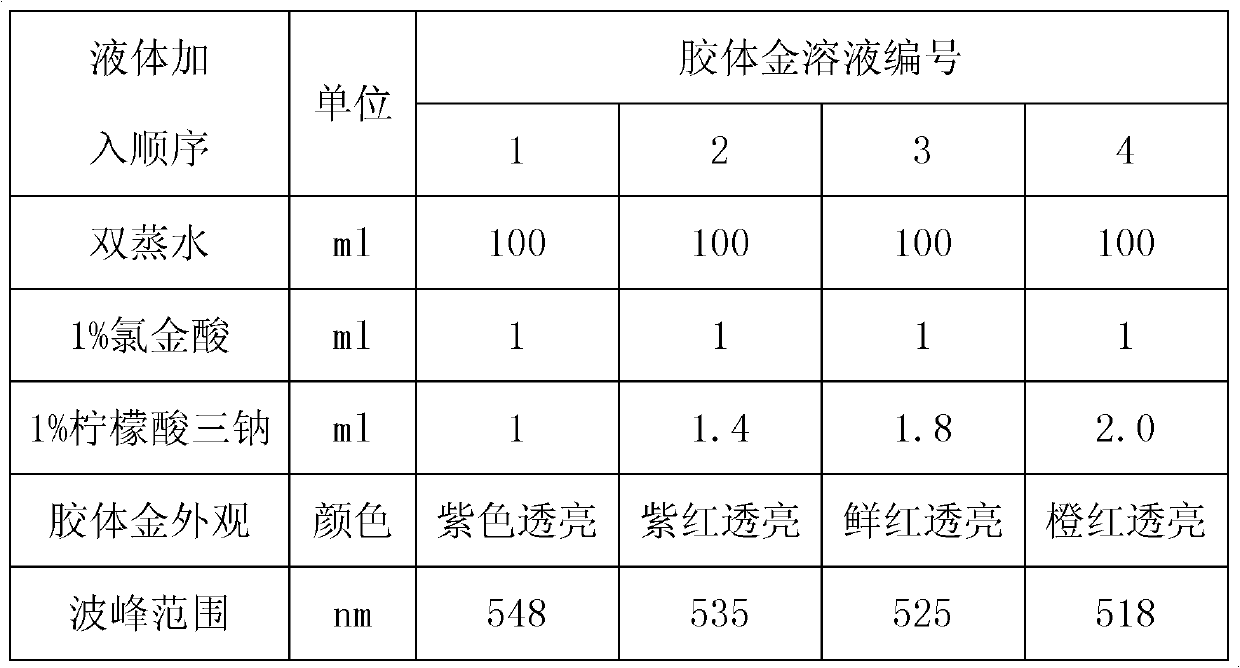
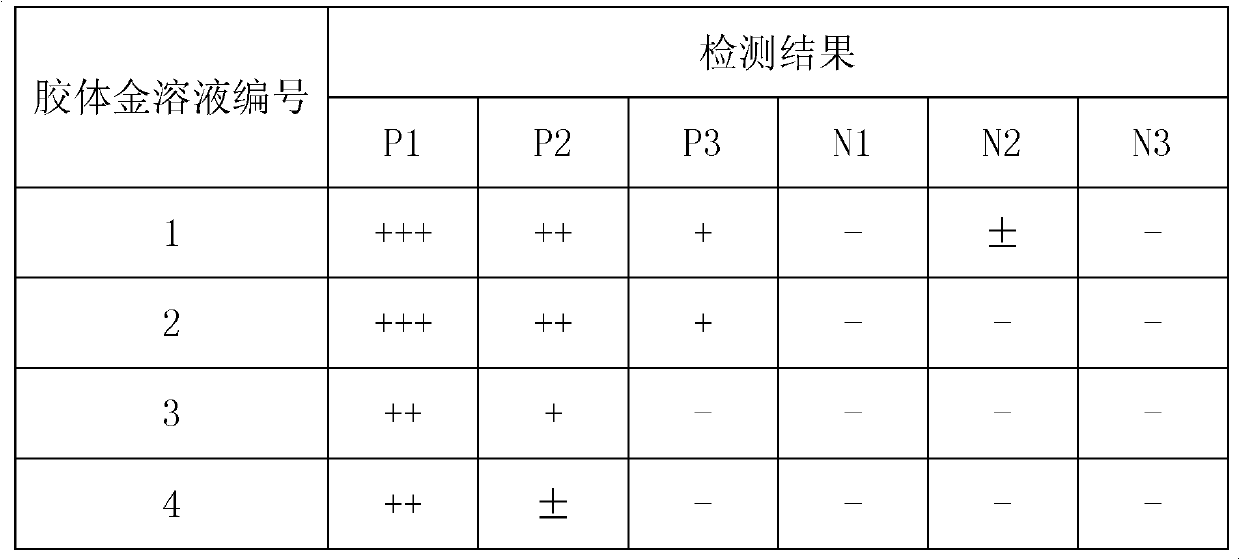
[0134] Embodiment 2: the selection of reaction time and sample amount, embodiment is the same as result as shown in table 16.
[0135] Table 16:
[0136]
[0137] Result analysis: From the experimental results in Table 16, it can be seen that the amount of sample added will affect the correct reaction result of the test strip. If the amount of sample added is low, there will be missed detection, and when the amount of sample added is high, false positives will easily occur. Based on the above experimental results, considering the stability of the test results and the convenience of operation, the sample volume was selected to be 10 μl of serum, followed by 100 μl of sample diluent, and 15-25 minutes for interpretation.
Embodiment 3
[0138] Embodiment 3: sample detection
[0139] (1) Preparation of sample diluent (according to 100ml volume):
[0140] Weigh 0.06g of NaH 2 PO 4 2H 2 O and 0.58g Na 2 HPO 4 12H 2 O, add 80.0mL of purified water, stir to fully dissolve, then add 0.85g of NaCl, mix well, set the volume to 100mL, measure the pH value to 7.30-7.50, store at 4-30°C for later use, and the validity period is 14 months.
[0141] (2) Collection and storage of testing samples:
[0142] Serum samples were collected intravenously according to routine methods. Samples determined within 5 days can be stored at 4°C. Samples can be stored at 20°C for at least 3 months. Samples should avoid hemolysis or repeated freezing and thawing. Samples that are turbid or precipitated should be clarified by centrifugation or filtration before testing.
[0143] (3) Detection:
[0144] Take out the reagent card from the original packaging aluminum foil bag, place it flat on the horizontal workbench and mark the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com