Prophylactic and/or therapeutic agent for metabolic syndrome
A technology of metabolic syndrome and composition, which is applied in the field of functional food and health food, can solve problems such as increased risk, and achieve the effect of promoting prevention and/or treatment without hindering hyperglycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] 1. Preparation of test substances, etc.
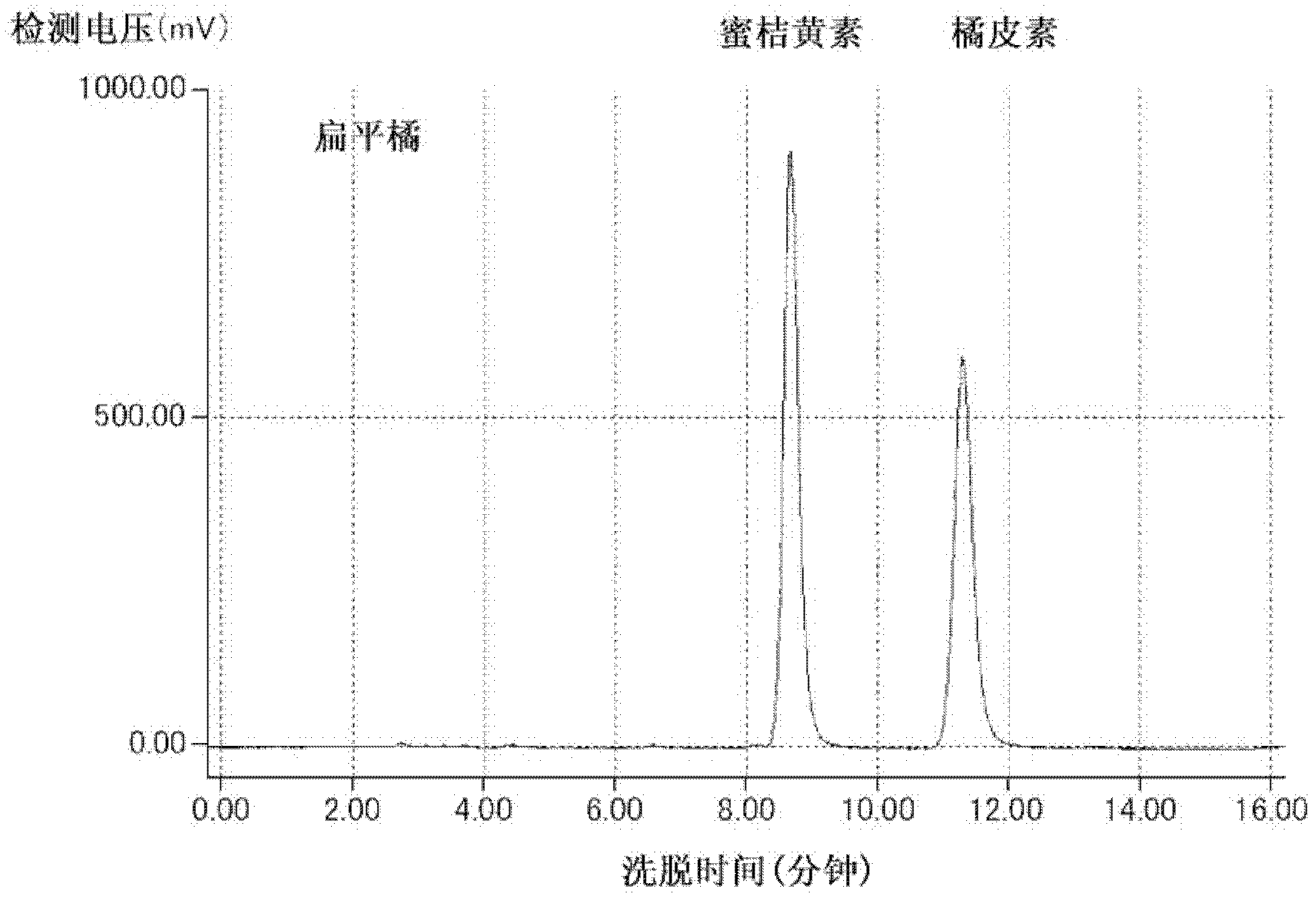
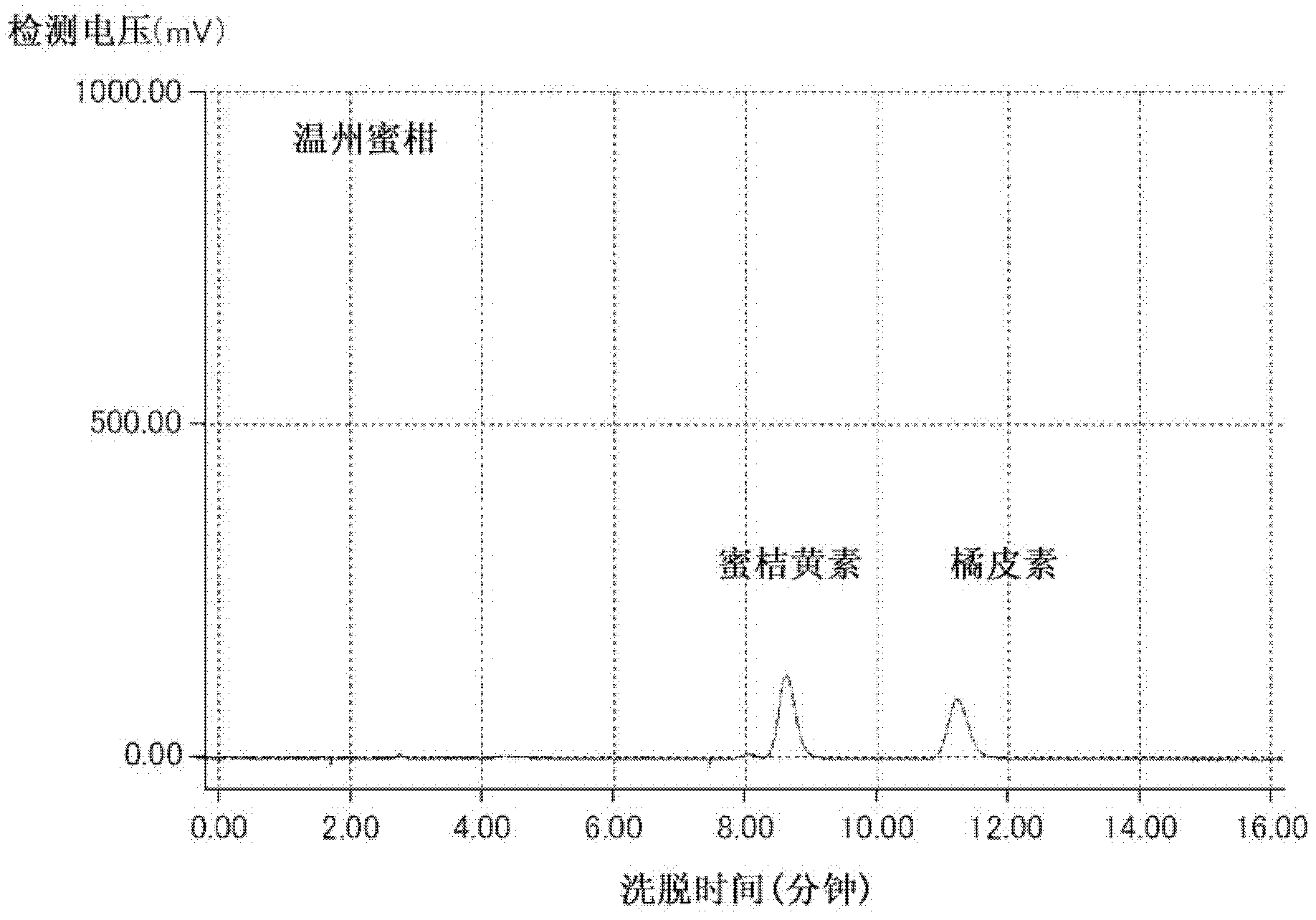
[0145] (1-1) The extraction method of the extract from the citrus peel of the mandarin orange region
[0146] The fruit of the flat mandarin orange is purchased from Daigimi, Okinawa Prefecture. The peel obtained from the flat orange fruit was dried in the sun for 3 days to obtain the flat orange dried peel. Satsuma tangerines are purchased from Ehime prefecture. In addition, the pericarp obtained from Satsuma citrus was dried in the sun for 3 days in the same manner to obtain dried satsuma citrus peel.
[0147] 40 L of methanol was added to 8 kg of these dried fruit peels to immerse them, and they were extracted at 4° C. for 14 days. Filtration was performed to obtain a crude extract, and the entire amount of the crude extract was concentrated using an evaporator. The concentrated solution was extracted by liquid-liquid distribution in 4 L of water / ethyl acetate (1 / 1), and then, the ethyl acetate phase was again extracted i...
Embodiment 2
[0163] 2. Measuring method of animal feed and gene expression level
[0164] (2-1) Feed for tested animals
[0165] CRF-1 (manufactured by Oriental Yeast Co., Ltd.) was purchased as a normal mouse feed (standard diet). As additives for the preparation of low-fat diet and high-fat diet, casein, tallow, β-cornstarch, α-cornstarch, dietary fiber, minerals and vitamins were purchased in the following amounts (w / w%) Add and use (both are manufactured by Oriental Yeast Co., ltd.). In addition, sucrose, L-cystine, choline bitartrate, and tert-butylhydroquinone were purchased and added to CRF-1 in the amounts (w / w%) shown in the table below for feeding (all were produced by Wako Pure Chemical Industry Co., Ltd. company manufacturing).
[0166] Table 1 is the food composition of the low-fat diet, the high-fat diet and the food added with SPE in Example 3. Each value represents weight % (w / w %). Components indicated by *1 were purchased from Wako Pure Chemical Industries. Sucrose,...
Embodiment 3
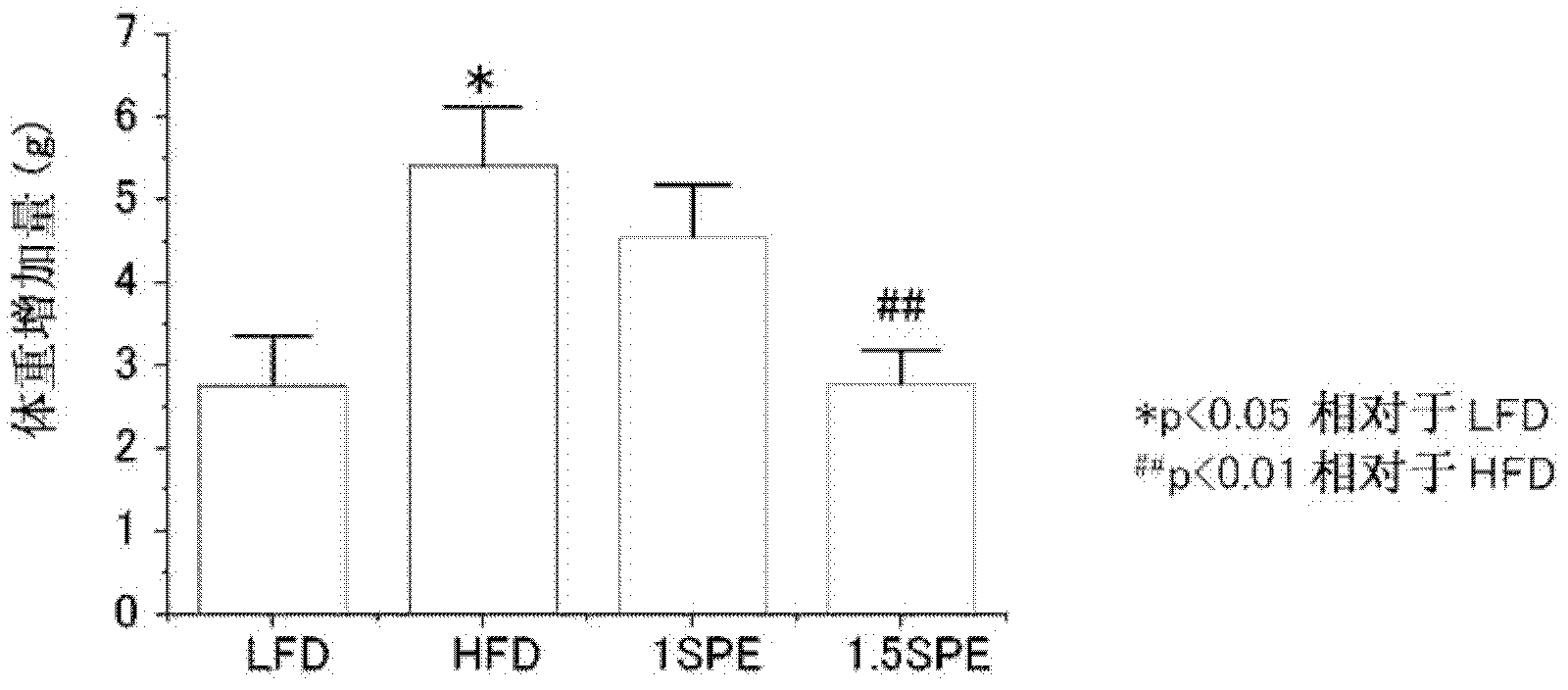
[0173] 3. Determination of the effect of SPE on obesity during high-fat diet intake
[0174] Animals used in the following examples were provided with a low fat diet (4% fat content) or a high fat diet (40% fat content). As a high-fat diet, three types of SPE-free diet, 1% SPE-added diet, and 1.5% SPE-added diet were prepared. In Example 3 below, the group taking a low-fat diet is represented as LFD intake group, the group taking SPE without high-fat diet is represented as HFD intake group, and the group taking 1% SPE plus high-fat diet is represented as HFD+1SPE intake In the group, the group that ingested 1.5% SPE was denoted as the HFD+1.5SPE ingestion group. In addition, in the following tables, they are represented as LFD, HFD, HFD+1SPE, and HFD+1.5SPE, respectively.
[0175] Regarding the animals used, C57BL / 6 mice at the age of 7 weeks after birth were purchased from CHARLES RIVER LABORATORIES JAPAN, INC. After being domesticated with standard food for 1 week, they we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com