Method utilizing witherite to prepare barium hydroxide octahydrate
A technology of barium hydroxide and barium witherite, applied in the direction of calcium/strontium/barium oxide/hydroxide, etc., can solve the problems such as low utilization rate of barium hydroxide, and achieve stable and reliable product quality, high decomposition efficiency, and easy operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
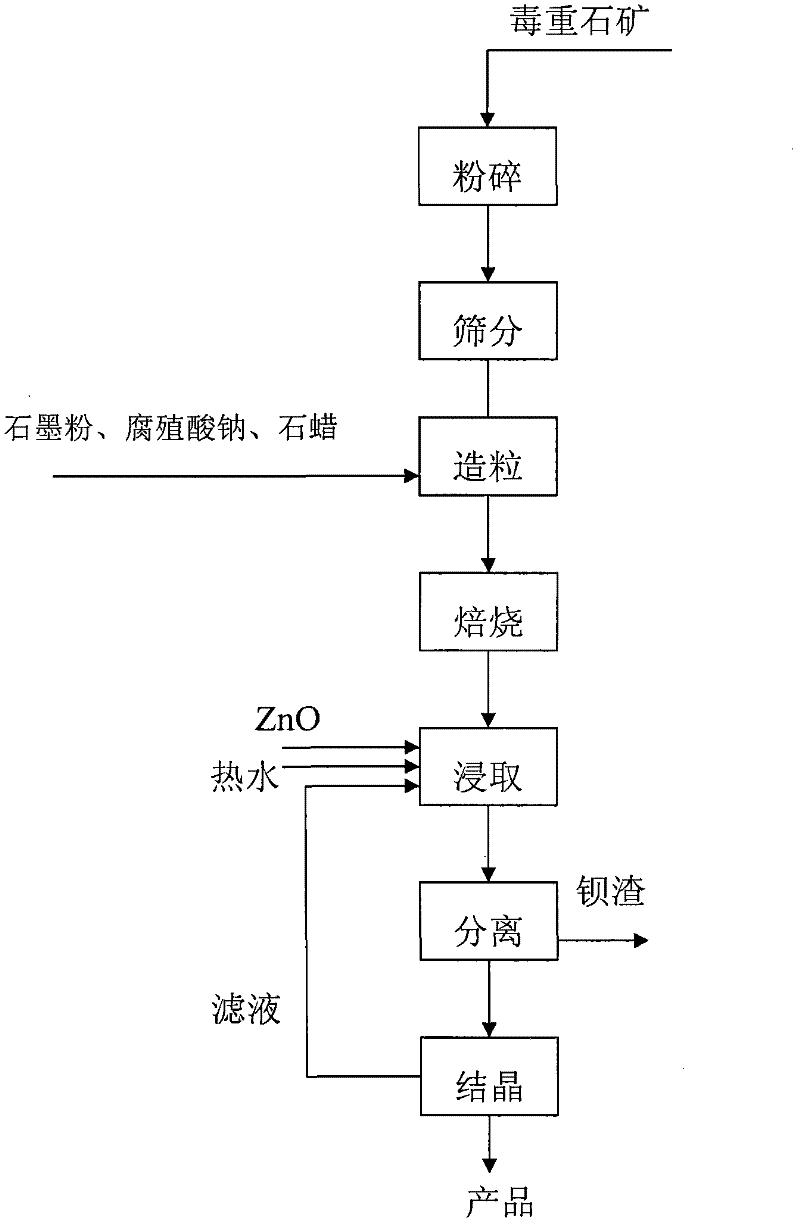
[0022] Example 1: barium hydroxide is produced from witherite barium ore, crushed, ground and sieved witherite barium ore to control the particle size range to 120 mesh > 80%, add graphite powder 8%, sodium humate 1%, paraffin wax 2%, mix Granulate evenly with a granulator. After the ball is dry and hard, put it into the corundum boat, heat it with the furnace, keep it at 1100°C for 3 hours, and after cooling down to 500°C, put the clinker into the leaching kettle, use 85°C hot water, according to the liquid-solid ratio 10:1, medium speed leaching for 1 hour. After desulfurization, heat and concentrate the obtained feed liquid to 0.8mol.L -1 , natural cooling and crystallization, separation and drying to obtain octahydrate barium hydroxide, after filtration, the raffinate is soaked back for irrigation.
example 2
[0023] Example 2: Barium hydroxide is produced from witherite barium ore, crushed, ground and sieved witherite barium ore to control the particle size range of 120 mesh > 60%, add graphite powder 10%, sodium humate 1.2%, paraffin 2.5%, mix Granulate evenly with a granulator. After the ball is dry and hard, put it into a corundum boat, heat it with the furnace, and keep it at 1150°C for 3 hours. After cooling down to 600°C, put the clinker into the leaching kettle, use 90°C hot water, and adjust the temperature according to the liquid-solid ratio. 8:1, medium speed leaching for 1.5 hours. After desulfurization, heat and concentrate the obtained feed liquid to 0.8mol.L -1 , natural cooling and crystallization, separation and drying to obtain octahydrate barium hydroxide, after filtration, the raffinate is soaked back for irrigation.
example 3
[0024] Example 3: barium hydroxide is produced from witherite barium ore, pulverized, ground and sieved witherite barium ore to control the particle size range to 120 mesh > 70%, add graphite powder 15%, sodium humate 2%, paraffin wax 3%, mix Granulate evenly with a granulator. After the ball is dry and hard, put it into a corundum boat, heat it with the furnace, and keep it at 1200°C for 1 hour. After cooling down to 700°C, put the clinker into the leaching kettle, use 95°C hot water, and adjust the temperature according to the liquid-solid ratio. 7:1, medium speed leaching for 2 hours. After desulfurization, heat and concentrate the obtained feed liquid to 0.8mol.L -1 , natural cooling and crystallization, separation and drying to obtain octahydrate barium hydroxide, after filtration, the raffinate is soaked back for irrigation.
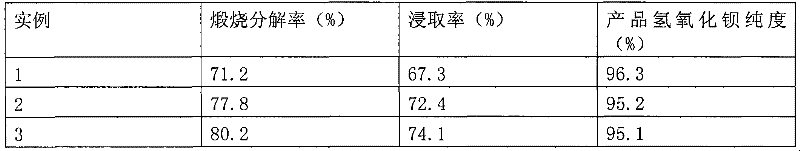
[0025] Experimental results
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com