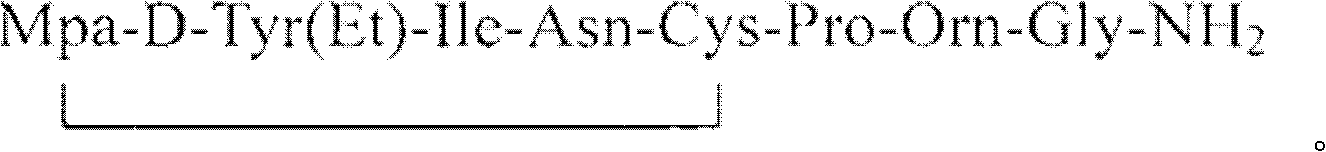Purification method for atosiban
A purification method and solution technology, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as failure to meet industrialization requirements, low peptide purity, and low application value, and achieve simple and feasible operation, The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
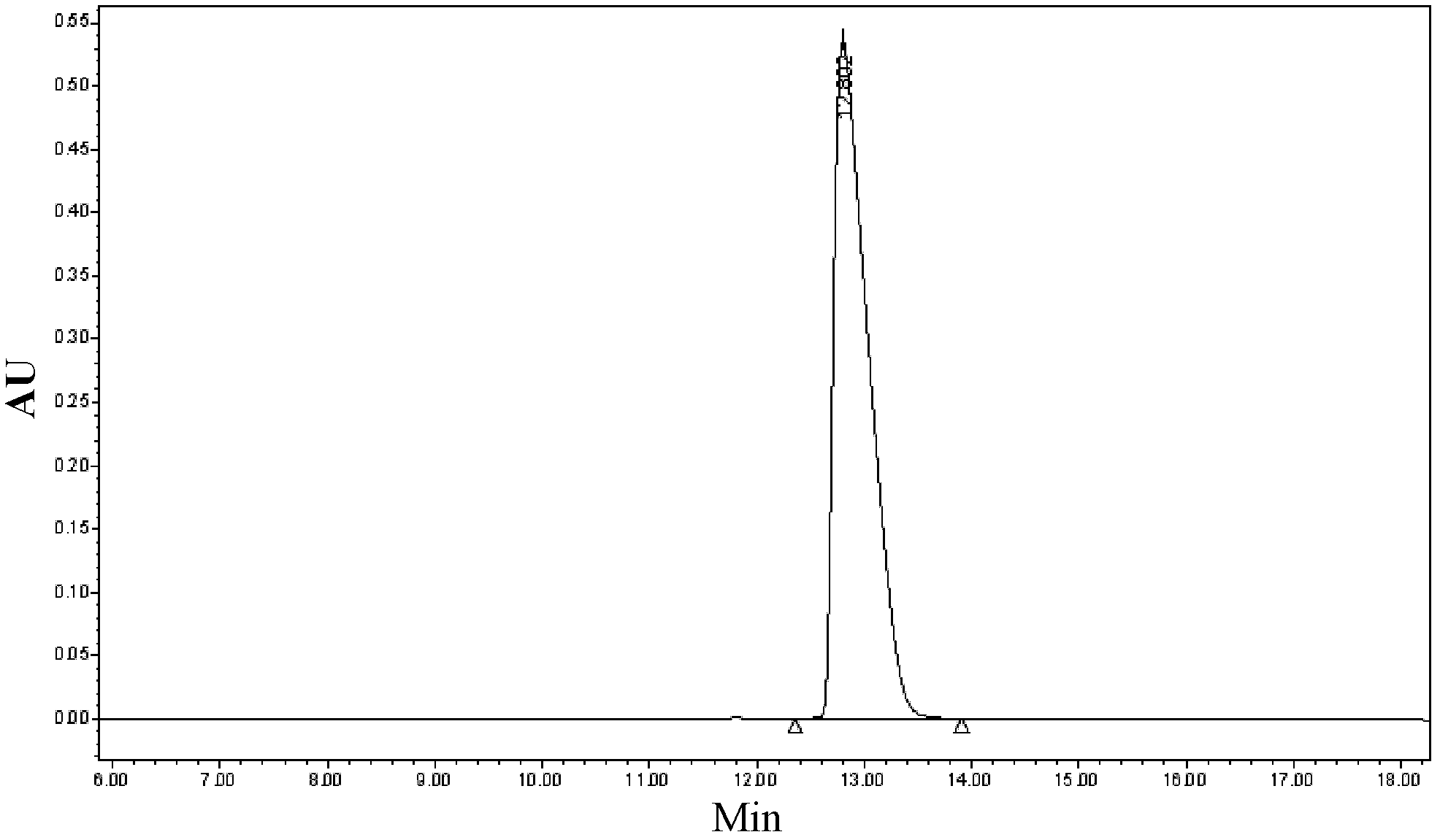
[0036] Dissolve the crude peptide at a concentration of 100 g / L with 50% acetic acid aqueous solution by volume, stir to dissolve the sample completely, filter it with a filter membrane, and collect the filtrate. Add ferric chloride reagent according to the proportion of 5% of the weight of the crude peptide, and dilute the volume ratio of the acetic acid in the crude peptide solution to 40% with water for later use.
[0037] A chromatographic column with octadecylsilane bonded silica gel as the stationary phase, the chromatographic column is: 5cm×25cm (diameter×length). Rinse the chromatographic column with more than 50% acetonitrile, and then equilibrate the sample, and the sample load is 1.5-3g. The mixed solution with perchlorate aqueous solution and acetonitrile is mobile phase, and wherein mobile phase A phase is 0.4% perchloric acid aqueous solution (v / v), adjusts pH value 2.5 with the sodium hydroxide aqueous solution of 50mmol; Mobile phase B phase is Acetonitrile. Th...
Embodiment 2
[0040] Dissolve the crude peptide at a concentration of 100 g / L with 80% acetic acid aqueous solution by volume, stir to dissolve the sample completely, filter it with a filter membrane, and collect the filtrate. The ferric sulfate reagent is added according to the ratio of 4% of the weight of the crude peptide, and the volume ratio of the acetic acid in the crude peptide solution is diluted to 30% by water for use.
[0041] A chromatographic column with octaalkylsilane bonded silica gel as the stationary phase, the chromatographic column is: 15cm×25cm (diameter×length). Rinse the chromatographic column with more than 50% acetonitrile, and then balance the sample, and the sample load is 25-40g. With the mixed solution of perchlorate aqueous solution and acetonitrile as mobile phase, wherein mobile phase A phase is 0.3% perchloric acid aqueous solution (v / v), adjust pH value 3.2 with the potassium hydroxide aqueous solution of 50mmol; Mobile phase B phase is Acetonitrile. The...
Embodiment 3
[0044] Dissolve the crude peptide at a concentration of 100 g / L with 90% acetic acid aqueous solution by volume, stir to dissolve the sample completely, filter it with a filter membrane, and collect the filtrate. Add ferric chloride reagent according to the ratio of 8% of the weight of the crude peptide, and dilute the volume ratio of the acetic acid in the crude peptide solution to 40% with water for later use.
[0045] A chromatographic column with octadecylsilane bonded silica gel as the stationary phase, the chromatographic column is: 30cm×25cm (diameter×length). Rinse the chromatographic column with more than 50% acetonitrile, and then equilibrate the sample, and the sample load is 80-170g. With the mixed solution of perchlorate aqueous solution and acetonitrile as mobile phase, wherein mobile phase A phase is 0.8% perchloric acid aqueous solution (v / v), adjust pH value 3.0 with the potassium hydroxide aqueous solution of 50mmol; Mobile phase B phase is Acetonitrile. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com