Fusion protein containing glicetin-1 as well as preparation method and application
A technology of glucagon and fusion protein, which is applied in the field of fusion protein containing glucagon-like peptide-1 and its preparation, to achieve the effects of stimulating secretion, improving glucose tolerance, and protecting pancreatic beta cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
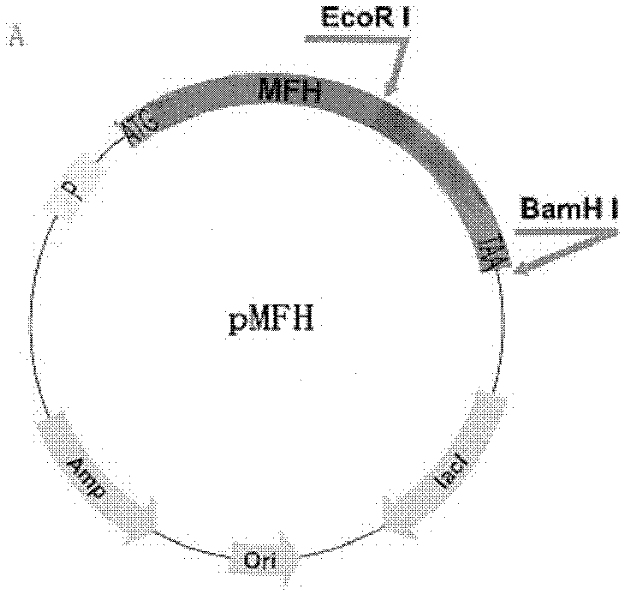
[0045] A recombinant plasmid containing the sequence encoding the fusion protein of glucagon-like peptide-1 or its derivatives (abbreviated as GLP-1 fusion protein) was constructed.
[0046] (1) PCR and enzyme digestion of the nucleotide sequence encoding the GLP-1 fusion protein
[0047] According to the structural characteristics of the GLP-1 fusion protein, the codon preference of Escherichia coli was used to design primers, and the primers were commercially synthesized by Shanghai Bioengineering Co., Ltd.
[0048] The primer sequences are as follows:
[0049] Upstream Primer 1 (UP1): 5'-GCGC GAT CCG CTG CCG CAT AGCCAT CGC GCC CAT AGC CTG CCG CCG-3’
[0050] Upstream primer 2 (UP2): 5'-CAT AGC CTG CCG CCG TTC AAC CCG AAG ACGCCG CAC GCT GAA GGT ACC-3'
[0051] Downstream primer (DP): 5'-TCATCCGCCAAAACAGCCAAG-3';
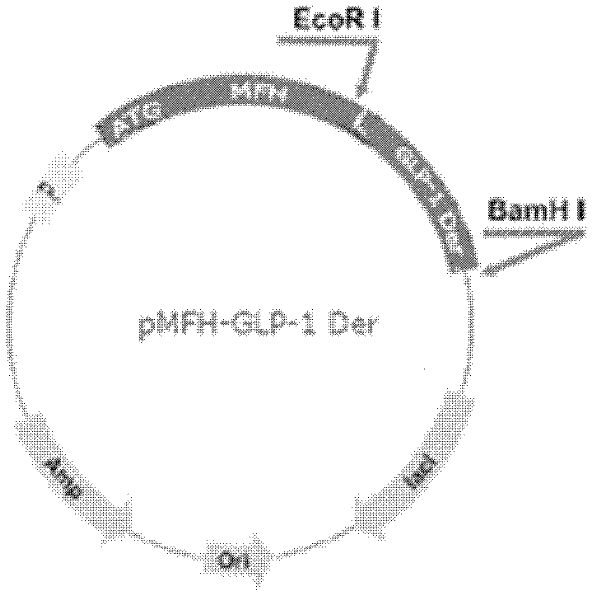
[0052] Using the overlap extension PCR method, the plasmid pMFH-GLP-1(7-37) (this plasmid has been applied for an invention patent application with the applic...
Embodiment 2
[0072] Example 2: Fermentation expression of pMFH-GLP-1 fusion protein
[0073] The recombinant plasmid pMFH-GLP-1Der was extracted, and further transformed into Escherichia coli BL21(DE3) (Bao Biology (Dalian) Bioengineering Co., Ltd.) to obtain genetically engineered expression bacteria. Through shaking flask culture and gradually amplifying, culture and ferment GLP-1 fusion protein expressing bacteria in 5L fermenter. The medium used is 2YT medium, the formula is: tryptone 16g / L, lactose 10g / L, NaCl 5g / L; use NaOH to adjust the pH to 7.0, and sterilize in the fermenter. The sterilization conditions are: 121°C, 20min . The fermentation culture conditions are as follows: the inoculum size is 1% by volume; the culture temperature is 37° C.; the liquid filling volume is 80% by volume; the concentration of ampicillin (Amp) is 100 mg / L. Cultivate to the middle and late stages of logarithmic growth, add 0.6mM IPTG, induce time for 6h, and collect the bacteria by centrifugation a...
Embodiment 3
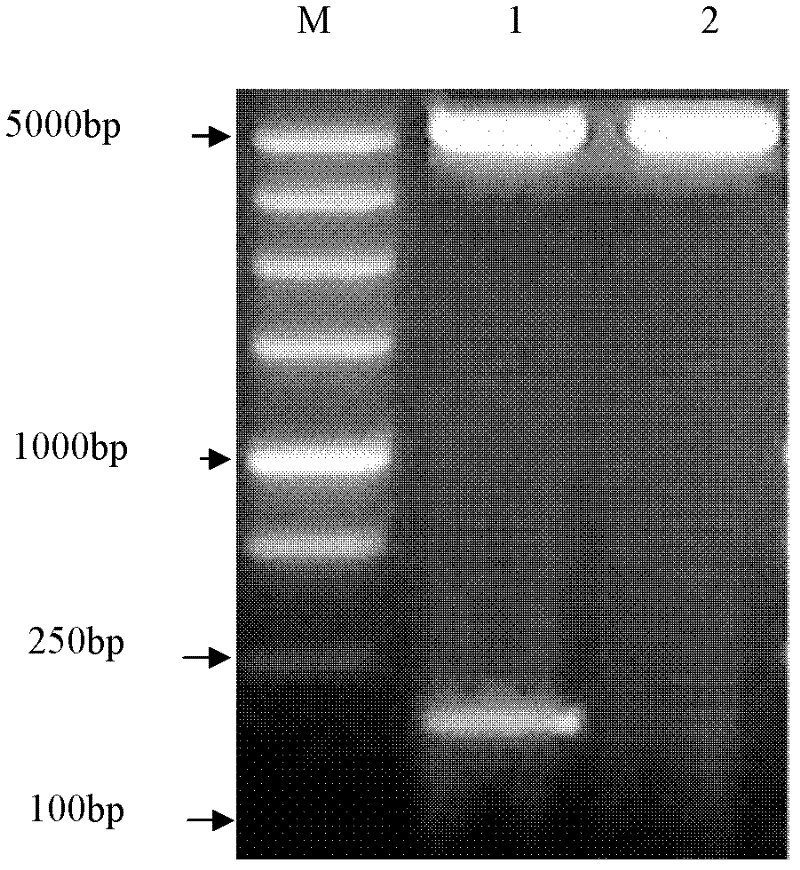
[0074] Example 3: Separation and purification to obtain GLP-1 fusion protein
[0075] (1) Collect the fermented cells by centrifugation and resuspend with lysate. The formula of lysate is: 50mmol / LNaH 2 PO 4 , 300mmol / L NaCL, 10mmol / L imidazole (imidazole), adjust the pH to 8.0 with NaOH. Ultrasonic disruptor (DF-6P3C Ultrasonic Cell Disruptor, Ningbo Xinzhike Research Institute) was used to disrupt the bacterial cells in an ice bath, turned on for 5 s, stopped for 5 s, and worked for 20 min. 10000rpm, 4°C, 30min, centrifuge to break up the bacteria, and collect the supernatant.
[0076] (2) Purify the supernatant collected in step (1) with a Ni-NTA Agarose (nickel-charged resin, QIAGEN) affinity column, wash away impurity proteins with a lysate containing 10mM imidazole, and then use a lysate containing 250mM imidazole The lysate (except for the different imidazole concentration, other components are the same as the above lysate) elutes the target protein.
[0077] (3) Hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com