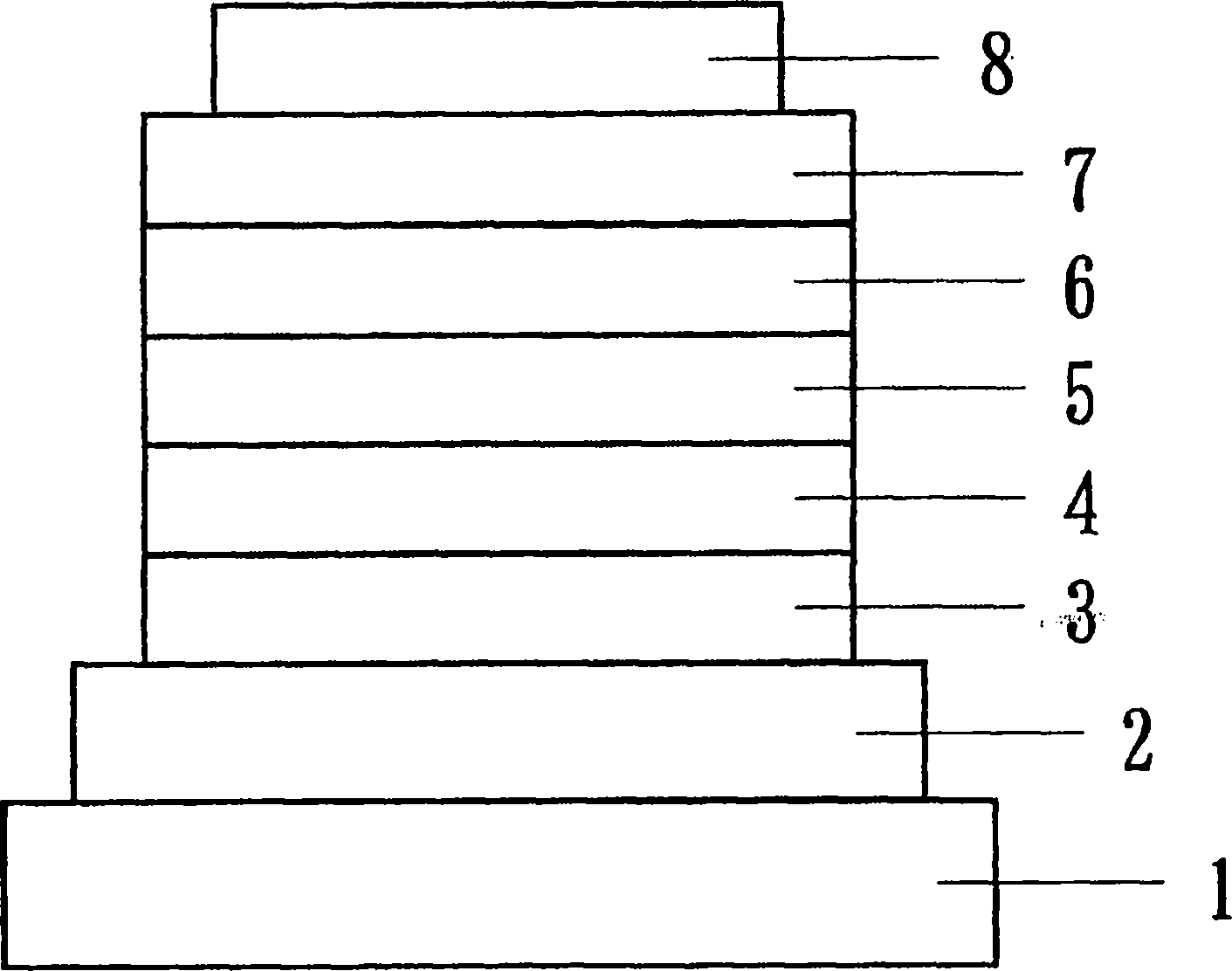
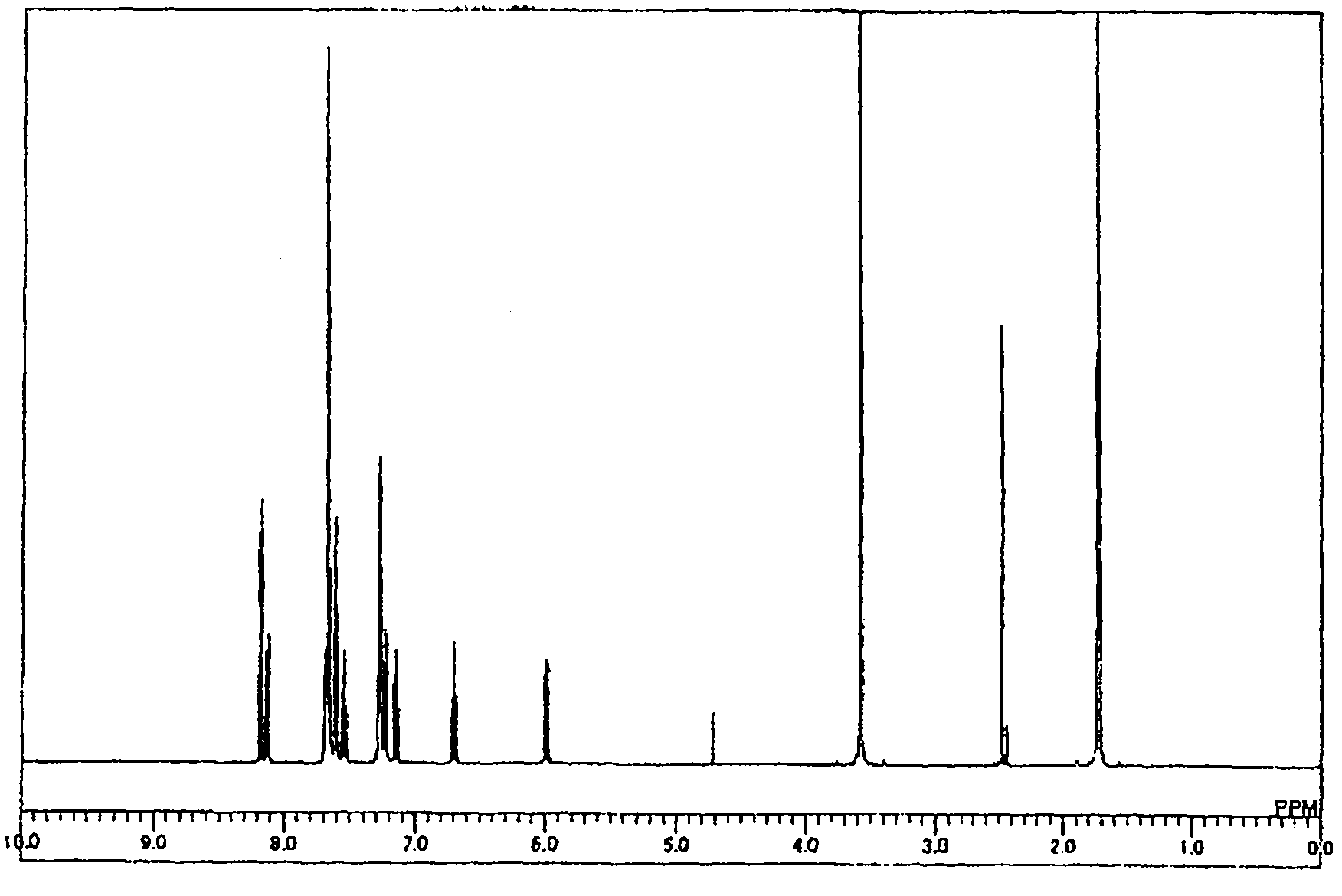
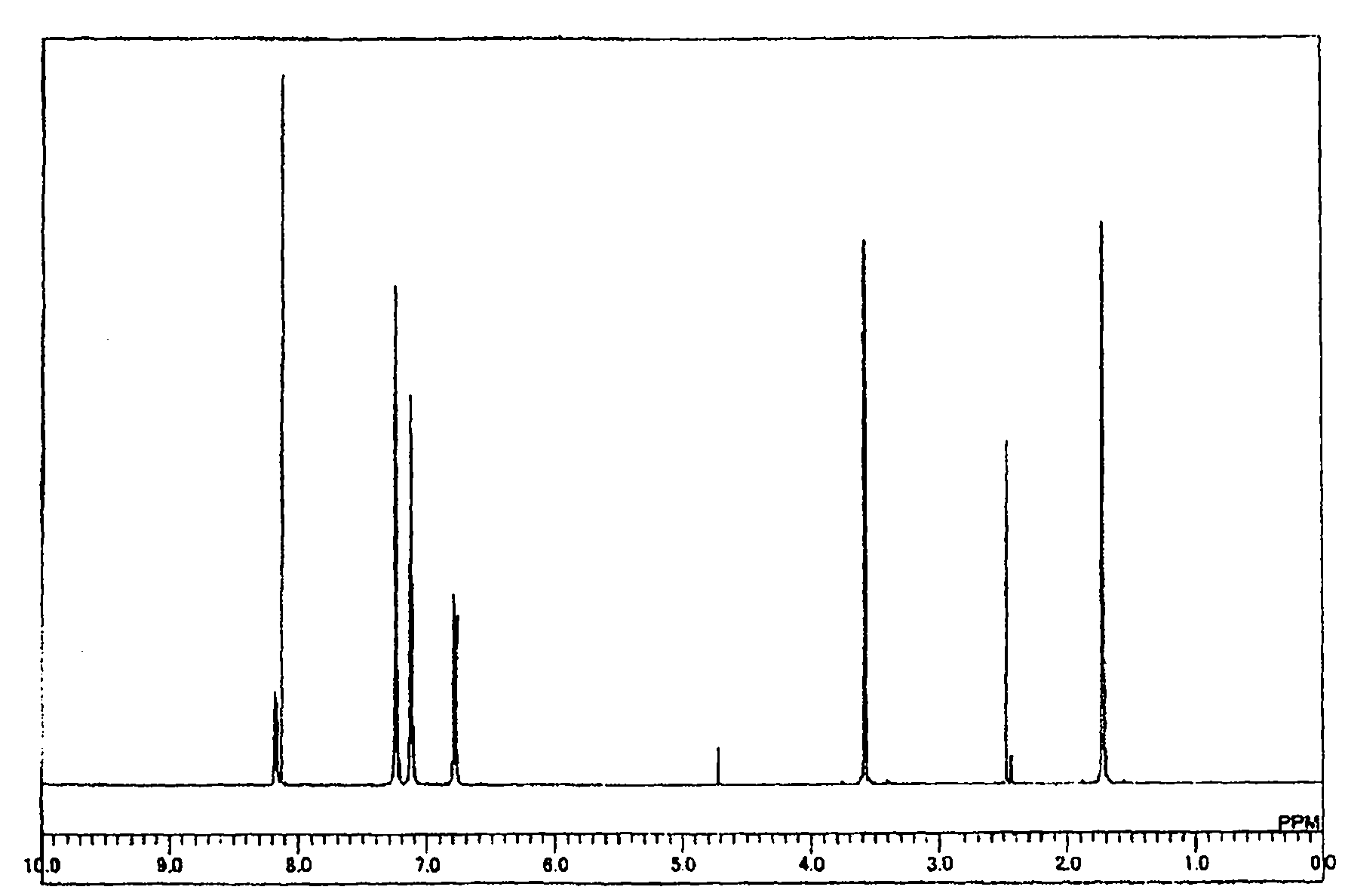
Organic electroluminescent element
A luminescent and organic field technology, applied in the field of organic electroluminescent components, can solve the problems of high driving voltage, poor life characteristics, and fully satisfied practical aspects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0146] Hereinafter, the present invention will be described in more detail using examples, but the present invention is of course not limited to these examples, and can be implemented in various forms as long as the gist of the present invention is not exceeded.
[0147] Synthesis examples of the compounds of the present invention are shown below. It should be noted that the compound number corresponds to the number carried in the above chemical formula.
Synthetic example 1
[0149] Synthesis of compound 1-1
[0150]
[0151]Under a nitrogen atmosphere, while stirring a solution of 20.0 g (0.17 mol) of indole in 300 ml of dehydrated diethyl ether at room temperature, 112.0 g of concentrated hydrochloric acid ( 1.10mol) to produce hydrogen chloride gas. After stirring the reaction solution at room temperature for 15 hours, 121.0 g of ethyl acetate and 303.2 g of saturated aqueous sodium bicarbonate solution were added. After the aqueous layer was extracted with ethyl acetate (2×100 ml), the organic layer was washed with saturated aqueous sodium bicarbonate (100 ml) and distilled water (2×100 ml). After drying the organic layer with anhydrous magnesium sulfate, the magnesium sulfate was filtered off, and the solvent was distilled off under reduced pressure. The obtained residue was dissolved in 150 ml of toluene, and after adding 2.5 g of palladium / activated carbon, the mixture was stirred for 3 hours while heating to reflux at 111°C. After coo...
Synthetic example 2
[0158] Synthesis of compound 2-1
[0159]
[0160] Under a nitrogen atmosphere, 33.3 g (0.30 mol) of 1,2-cyclohexanedione, 86.0 g (0.60 mol) of phenylhydrazine hydrochloride, and 1,000 ml of ethanol were stirred at room temperature, and concentrated sulfuric acid was added dropwise over 5 minutes. After 3.0 g (0.031 mol), it stirred for 4 hours, heating at 65 degreeC. After cooling the reaction solution to room temperature, the precipitated crystals were collected by filtration and washed with ethanol (2×500 ml) to obtain 80.0 g of purple-brown crystals. 72.0 g (0.26 mol) of this crystal, 72.0 g of trifluoroacetic acid, and 720.0 g of acetic acid were stirred while heating at 100° C. for 15 hours. After cooling the reaction solution to room temperature, the precipitated crystals were collected by filtration and washed with acetic acid (200 ml). Reslurry purification was performed to obtain 30.0 g of Intermediate C as white crystals (yield 45%).
[0161]
[0162] Under...
PUM
| Property | Measurement | Unit |
|---|---|---|
| coating thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com