Preparation method of polysaccharide molecule fragment composite coating
A composite coating and fragment technology, applied in the direction of coatings, catheters, packaging items, etc., can solve the problems of poor anticoagulant activity and poor biocompatibility, and achieve the effects of low cost, improved degradation performance, and low natural toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
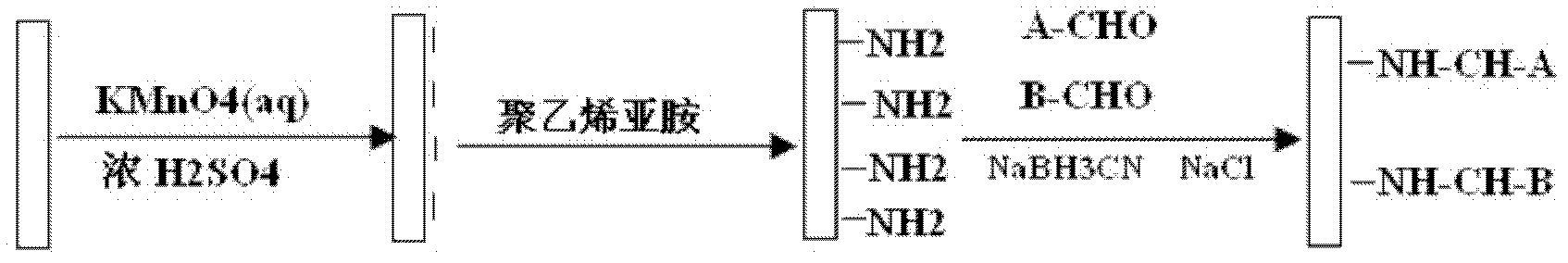
[0029] In the first step, 0.344 g of potassium permanganate was dissolved in 162 ml of deionized water, and 10 ml of 95 wt % concentrated sulfuric acid was slowly added to mix the concentrated sulfuric acid and potassium permanganate evenly.
[0030] In the second step, the polymer material PVC is added to the solution obtained in the first step, and the reaction is fully stirred for 10 minutes.
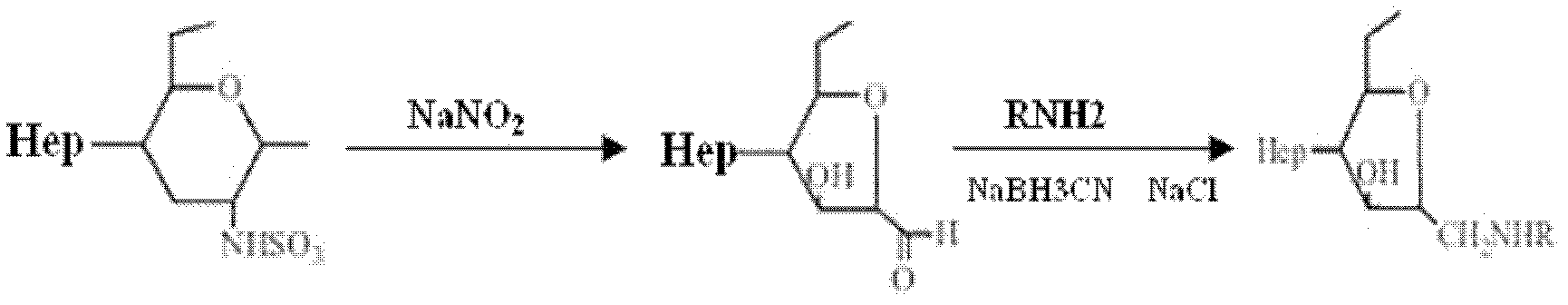
[0031] In the third step, 2 g of heparin sodium was dissolved in 200 ml of deionized water, 20 mg of sodium nitrite was added, and diazotization was performed at 0° C. for 1.5 hours. Afterwards, the pH of the reaction solution was adjusted to 7.0 to terminate the reaction, and the polysaccharide fragment A was obtained by dialysis through a 7000Da dialysis bag and freeze-drying at -80°C.
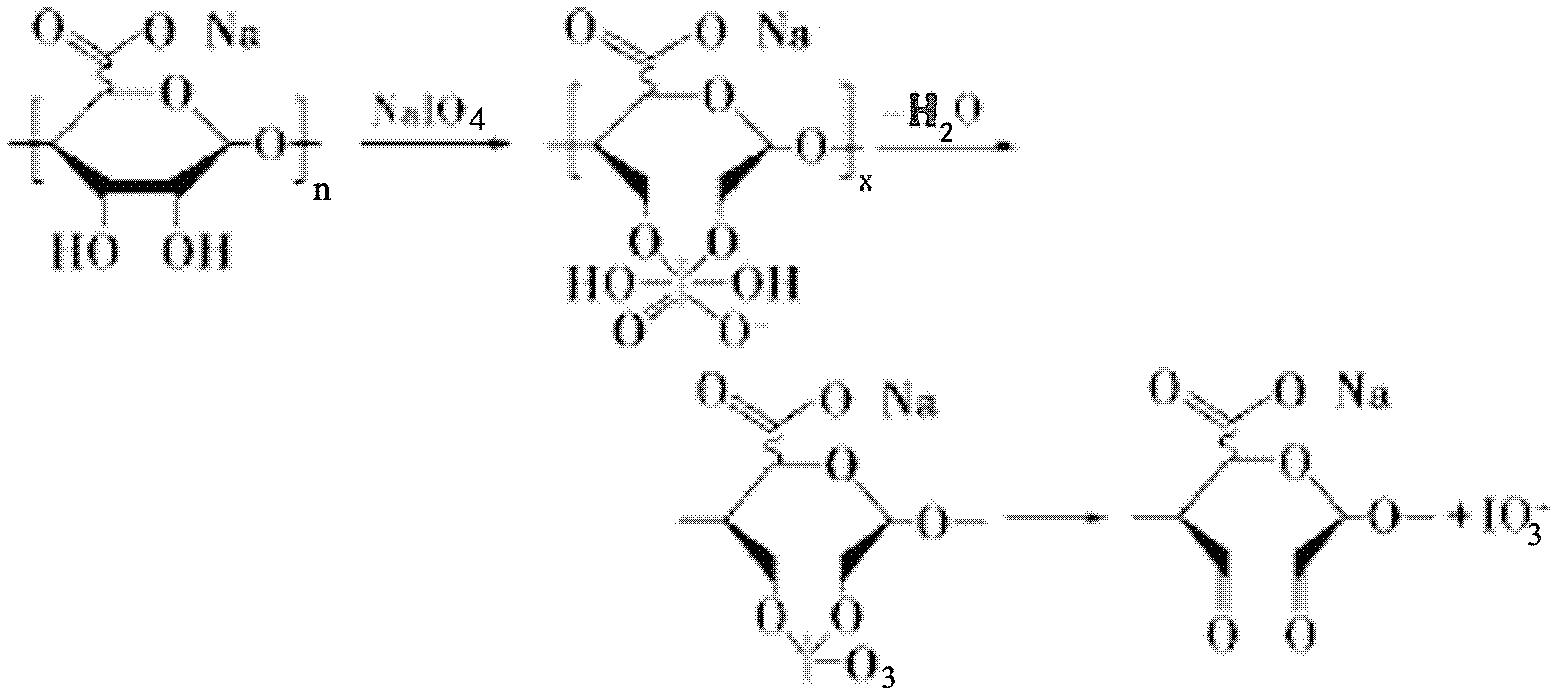
[0032] The fourth step is to oxidize the alginic acid according to the molar ratio of sodium periodate to sodium alginate unit of 1:10, so that the end of the alginic acid fragment is exposed to al...
Embodiment 2
[0037] In the first step, 0.328 g of potassium permanganate was dissolved in 144 ml of deionized water, and 20 ml of 95% concentrated sulfuric acid was slowly added to mix the concentrated sulfuric acid and potassium permanganate evenly.
[0038] In the second step, the polymer material is added to the solution obtained in the first step, and the mixture is stirred and fully reacted for 1 min.
[0039] In the third step, 2 g of sodium heparin was dissolved in 200 ml of deionized water, 20 mg of sodium nitrite was added, and diazotization was performed at 0° C. for 2 hours. Afterwards, the pH of the reaction solution was adjusted to 7.0 to terminate the reaction, and the polysaccharide fragment A was obtained by dialysis through a 7000Da dialysis bag and freeze-drying at -80°C.
[0040] The fourth step is to oxidize the alginic acid according to the molar ratio of sodium periodate and sodium alginate at 3:10, so that the end of the alginic acid fragments is exposed to aldehyde gr...
Embodiment 3
[0044] In the first step, 0.328 g of potassium permanganate was dissolved in 144 ml of deionized water, and 20 ml of 95% concentrated sulfuric acid was slowly added to mix the concentrated sulfuric acid and potassium permanganate evenly.
[0045] In the second step, the polymer material is added to the solution obtained in the first step, and stirred and fully reacted for 8 minutes.
[0046] In the third step, 2 g of heparin sodium was dissolved in 200 ml of deionized water, 20 mg of sodium nitrite was added, and diazotization was performed at 0° C. for 1.5 hours. Afterwards, the pH of the reaction solution was adjusted to 7.0 to terminate the reaction, and the polysaccharide fragment A was obtained by dialysis through a 7000Da dialysis bag and freeze-drying at -80°C.
[0047] The fourth step is to oxidize the alginic acid according to the molar ratio of sodium periodate and sodium alginate unit of 1:10, so that the end of the alginic acid fragment is exposed to the aldehyde g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com