Multifunctional polyethylene glycol-dual vitamin E succinate derivative and application thereof in drug delivery
A polyethylene glycol, succinate technology, applied in the direction of drug delivery, pharmaceutical formulations, etc., can solve the problems of inefficiency, high toxicity, etc., and achieve the effects of high encapsulation efficiency, easy operation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
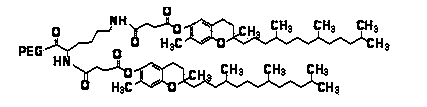
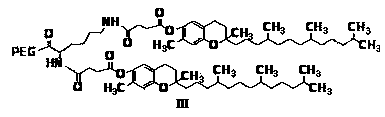
[0037] Example 1 Preparation of monomethoxy polyethylene glycol 2000-lysine-bis-vitamin E succinate block copolymer
[0038] (A) Dissolve 2.1 g of vitamin E succinate in dichloromethane, add 1 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.6 g of 1-Hydroxybenzotriazole, magnetically stirred at 0°C under ice bath for 1 h. 1.3 g benzyloxy lysine ester hydrochloride sulfonate was mixed with 3 mL triethylamine, and then added to the above activation solution, N 2 Magnetic stirring at 30°C for 24 h under protection. After the reaction is completed, wash and dry. After purification, benzyloxy lysine divitamin E succinate (structure formula I) is obtained.
[0039]
[0040] (B) Dissolve the product of step (a) in ethyl acetate, in palladium on carbon (Pd / C) and H 2 Under the action, magnetic stirring was carried out at 30℃ for 6 h. Filter, collect the filtrate, and rotate to obtain lysine divitamin E succinate (the structural formula is as formula II).
[0041...
Embodiment 2
[0045] Example 2 Preparation of polyethylene glycol 5000-lysine-bis-vitamin E succinate block copolymer
[0046] (A) Dissolve 2.1 g of vitamin E succinate in dichloromethane, add 1 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.6 g of 1-Hydroxybenzotriazole, magnetically stirred at 0°C in an ice bath for 1 h. 1.3 g benzyloxy lysine ester hydrochloride sulfonate was mixed with 3 mL triethylamine, and then added to the above activation solution, N 2 Magnetic stirring at 30°C for 24 h under protection. After the reaction is completed, wash and dry. After purification, benzyloxy lysine divitamin E succinate (structure formula I) is obtained.
[0047]
[0048] (B) Dissolve the product of step (a) in ethyl acetate, and stir magnetically at 30°C for 6 h under the action of palladium carbon and hydrogen. Filter, collect the filtrate, and rotate to obtain lysine divitamin E succinate (the structural formula is as formula II).
[0049]
[0050] (C) 1.2g Step (b)...
Embodiment 3
[0053] Example 3 Preparation of monomethoxy polyethylene glycol amidation-lysine-bis-vitamin E succinate block copolymer
[0054] (A) Dissolve 2.1 g of vitamin E succinate in dichloromethane, add 1 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) and 0.6 g of 1-hydroxybenzotriazole (HOBT) was stirred magnetically for 1 h in an ice bath at 0°C. 1.3 g benzyloxy lysine ester hydrochloride sulfonate was mixed with 3 mL triethylamine, and then added to the above activation solution, N 2 Magnetic stirring at 30°C for 24 h under protection. After the reaction is completed, wash and dry. After purification, benzyloxy lysine divitamin E succinate (structure formula I) is obtained.
[0055]
[0056] (B) Dissolve the product of step (a) in ethyl acetate, in palladium on carbon (Pd / C) and H 2 Under the action, magnetic stirring was carried out at 30℃ for 6 h. Filter, collect the filtrate, and rotate to obtain lysine divitamin E succinate (the structural formula is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com