High-dispersion fluorine-containing nanosphere and epoxy resin super-amphiphobic surface
A nano-microsphere, epoxy resin technology, applied in epoxy resin coatings, dyed polymer organic compound treatment, fibrous fillers, etc., can solve the problems of inability to disperse, weak adhesion, etc. The effect of weak adhesion and excellent hydrophobicity and oleophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
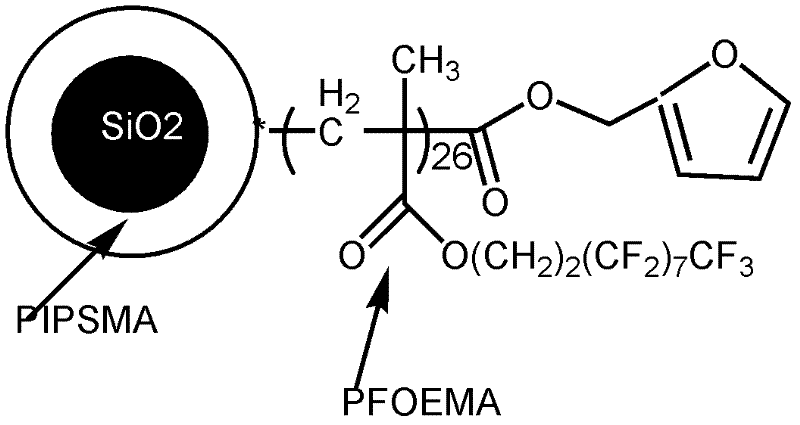
[0081] The end group is the preparation method of the fluoropolymer of compound D, comprises the following steps:
[0082] (1) Synthesis of furan ring initiator (furyl bromoisobutyrate)
[0083] Disperse 1.5g of furyl alcohol (furfuryl alcohol) in 30ml of anhydrous dichloromethane, add 4ml of triethylamine, slowly drop in 2ml of 2-bromoisobutyryl bromide under ice-water bath conditions, naturally warm up to room temperature and react for 4h, then Then wash it 3 times with saturated sodium bicarbonate solution, then wash it with pure water to neutrality, then dry it with anhydrous magnesium sulfate, remove dichloromethane, obtain a viscous liquid substance, and then distill under reduced pressure to obtain the furan ring Initiator.
[0084] The spectral analysis of the product is as follows: 1 H-NMR (CDCl 3 solvent): 7.30, 6.25, 6.19 (hydrogen on the furan ring, 3H), 5.19 (hydrogen on the methylene on furfuryl alcohol, 2H), 2.02 (hydrogen on the dibromoisobutyryl bromide, 6H...
Embodiment 2
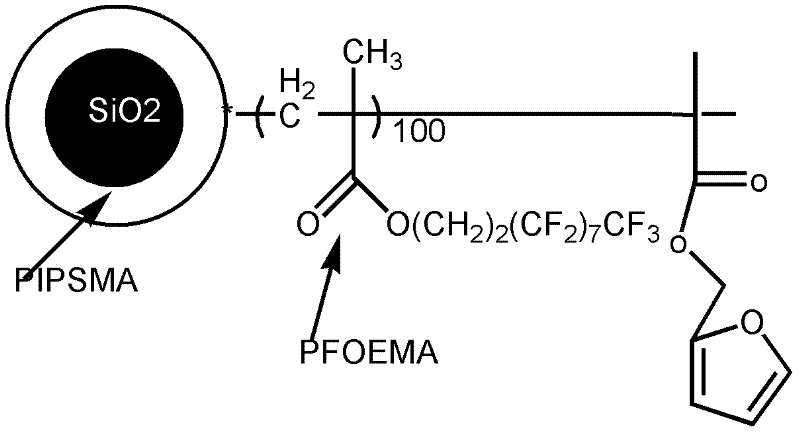
[0093] The end group is the preparation method of the fluoropolymer of compound D, comprises the following steps:
[0094] (1) Synthesis of furfuryl methacrylate
[0095] Disperse 1.23g of furyl alcohol (furfuryl alcohol) in 20ml of anhydrous tetrahydrofuran, add 2ml of triethylamine, slowly drop in 2.5ml of methacryloyl chloride under ice-water bath conditions, naturally warm up to room temperature and react for 24h, and then use saturated carbonic acid Wash it three times with sodium hydrogen solution, then wash it with pure water until neutral, then dry it with anhydrous magnesium sulfate, remove tetrahydrofuran, obtain a viscous liquid substance, and then distill under reduced pressure to obtain furfuryl methacrylate.
[0096] The spectral analysis of the product is as follows: 1 H-NMR (CDCl3 as solvent): 7.30, 6.25, 6.19 (hydrogen on the furan ring, 3H), 5.19 (hydrogen on the methylene on furfuryl alcohol, 2H), 1.78 (CH 3 , 3H); Infer that the product structure of the p...
Embodiment 3
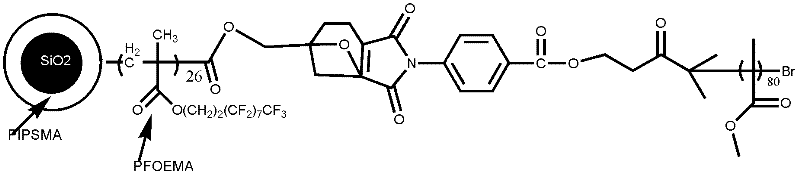
[0103] Adopt anionic polymer hair synthesis end group to be the highly dispersed polymer of compound A, comprise the following steps:
[0104] (1) Synthesis of ρ-CPMIC initiator
[0105] Add 30mL of dimethylformamide to a 500mL three-necked flask equipped with stirring, and simultaneously add a DMF solution of p-aminobenzoic acid solution (14g, 0.1mol) and maleic anhydride (11g, 0.11mol) dropwise. React at ~20°C for 2 hours to obtain yellow needle-like crystals. After filtration and drying, the intermediate product ρ-CPMA is obtained, with a melting point of 208-210°C;
[0106] Add ρ-CPMA (20g, 0.85mol), anhydrous sodium acetate (2g, 0.016mol), and acetic anhydride (48mL, 0.5mol) into a 250mL three-neck flask in sequence, keep it warm at 60-80°C for 1h, and cool down to room temperature , poured into 800mL ice water, precipitated, suction filtered, washed with water until neutral, recrystallized with 95% ethanol by volume fraction, and vacuum dried at 60°C to obtain ρ-CPMIC, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com