Efficient soluble expression and purification method of human LOX-1 (lectin like oxidized low density lipoprotein receptor-1) extracellular domain
An expression method and exogenous structure technology, applied in the field of efficient soluble expression and purification of human LOX-1 extracellular domain, can solve the problems of harsh reaction conditions, poor protein stability, time-consuming efficiency, etc., and achieve simple and efficient recovery and maintenance High stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 : Construction of expression vector and verification of soluble expression
[0055] 1. Construction of expression vector
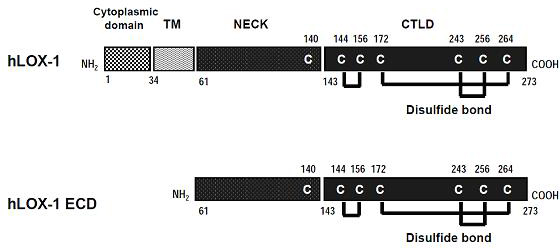
[0056] Select the hLOX-1 ECD domain as the expression object. The hLOX-1 ECD gene fragment was amplified from human LOX-1 cDNA by PCR reaction. Gateway technology is used to connect the target gene with the expression vector.
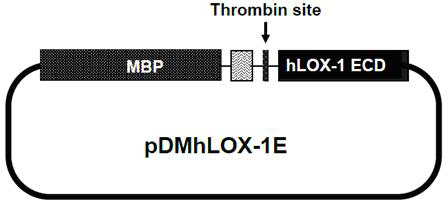
[0057] figure 2 is the model diagram of the constructed expression vector, wherein, MBP represents maltose binding protein; Thrombin site represents the thrombin cleavage site.
[0058] Specific steps are as follows:
[0059] 1. Using human LOX-1 cDNA as a template, design primers:
[0060] Primer 1: 5'-cacc CTGGTGCCACGCGGTTCT TCCCAGGTGTCTGACCTCC-3'
[0061] (The lowercase part is the guide base of the plasmid constructed by Gateway technology, and the underline indicates the thrombin recognition site)
[0062] Primer 2: 5'-tcattaCTGTGCTCTTAGGTTTGCCTTC-3'
[0063] (The lowercase part is the double s...
Embodiment 2
[0083] Example 2 : Establishment of large-scale expression and purification methods
[0084] Using the successfully constructed expression vector in Example 1, the expression conditions were optimized, and a purification method for the expressed protein was established.
[0085] The specific implementation plan is as follows:
[0086] 1. The plasmid pDMhLOX-1E was transformed into Escherichia coli Rosetta-gami2(DE3) by chemical transformation, expanded and stored for later use.
[0087] 2. Referring to Example 1, the expression conditions were optimized, and the culture volume was expanded to 1 liter. The specific implementation is as follows:
[0088] ⑴ Use a 250ml Erlenmeyer flask for pre-culture, with a liquid volume of 50ml, insert Escherichia coli and culture at 37 degrees to OD 600 = 0.8.
[0089] (2) Inoculate 3% of the inoculum into a 3 L shake flask containing 1 liter of medium, and continue to culture at 37 degrees until OD 600 = 0.5, followed by variable ...
Embodiment 3
[0108] Example 3 : Mass expression and purification of hLOX-1 ECD protein
[0109] On the basis of Examples 1 and 2, the feasibility of the invention for mass production of hLOX-1 ECD protein was verified by using a 5-liter fermenter.
[0110] The specific implementation plan is as follows:
[0111] 1. Fill 100 ml medium in a 500 ml Erlenmeyer flask as primary culture. Cultivate at 37 degrees to make the cell concentration reach OD 600 = 0.9.
[0112] 2. Inoculate 3% of the inoculum into a fermenter containing 2 liters of medium, stir at 120 rpm, adjust dissolved oxygen to 20%, and continue culturing at 37 degrees until OD 600 = 0.5-0.6. Then lower the temperature to 30 degrees, add IPTG with a final concentration of 300 micromolar to the culture medium, control the dissolved oxygen at 8-10%, and continue to cultivate until the cell concentration reaches OD 600 = 1.0-1.2, stop the fermentation and recover the bacteria.
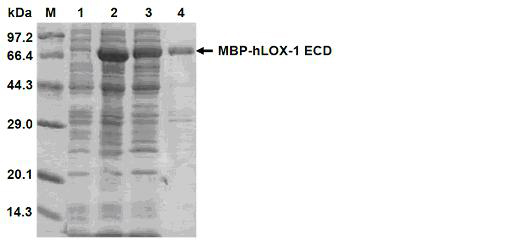
[0113] 3. Purify the expressed hLOX-1 ECD p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com