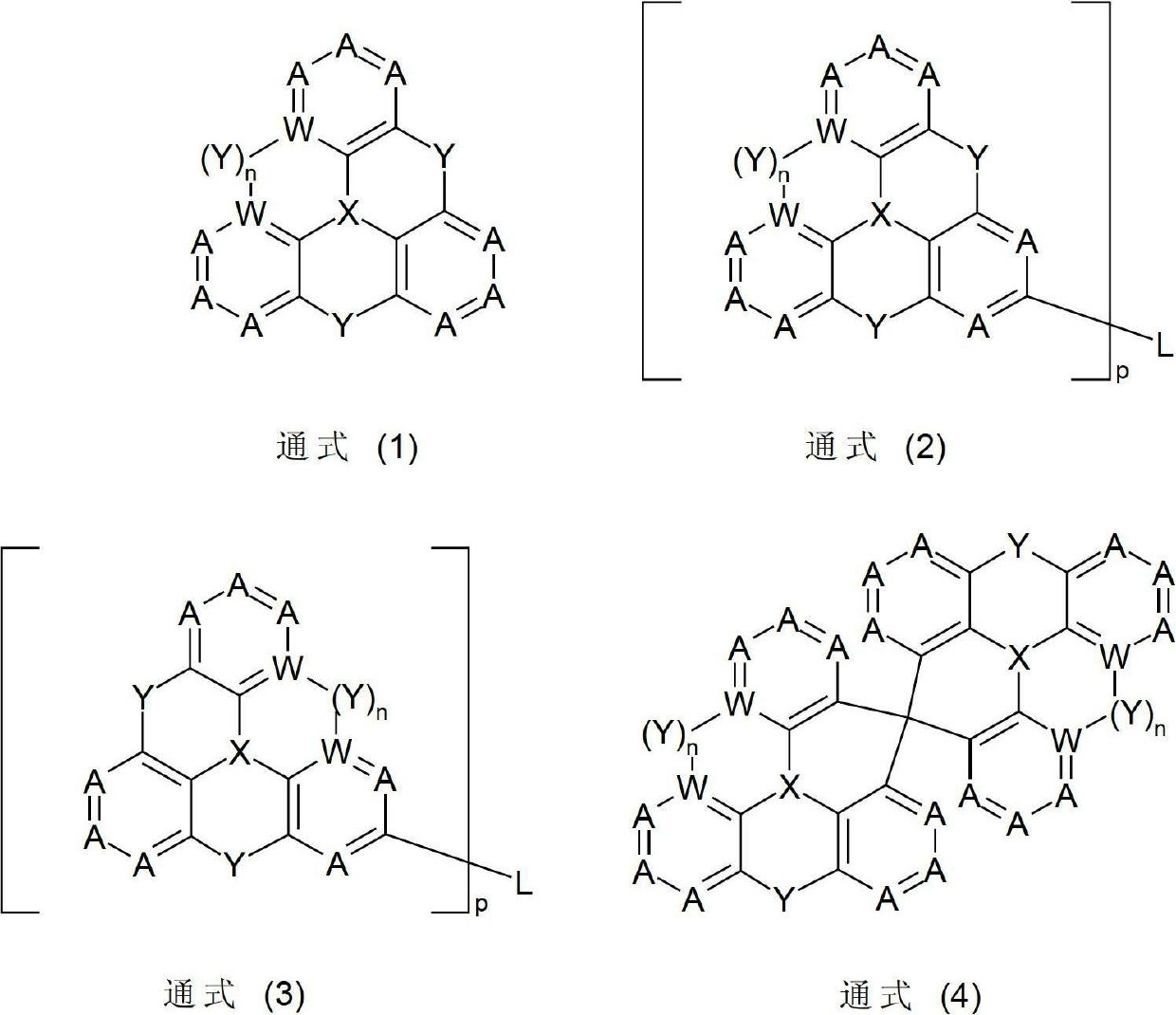
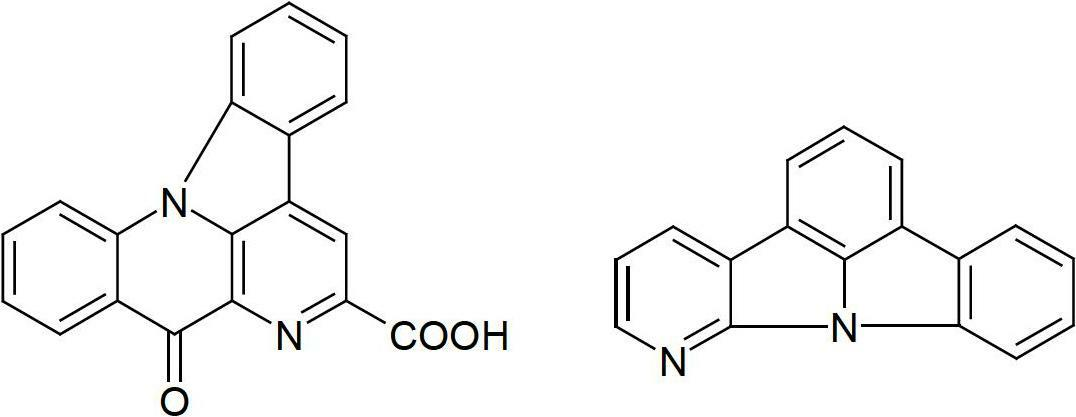
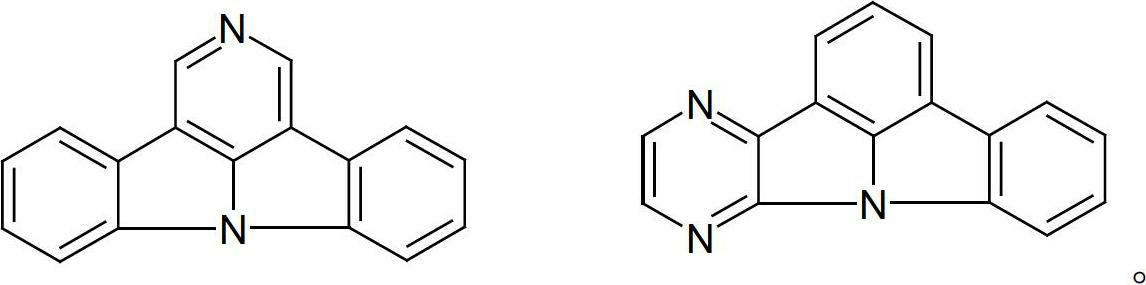
Nitrogen-containing condensed heterocyclic compounds for oleds
A technology of compounds and heteroaromatic rings, applied in the field of materials in organic electroluminescent devices, can solve the problems of no disclosure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212] Example 1: 6-bromo-8,8-dimethyl-8H-3,12b-diazabenzo[a]acethracene
[0213]
[0214] 1a) Methyl 2-pyrido[4,3-b]indol-5-ylbenzoate
[0215]
[0216] 16.8g (100mmol) of 5H-pyrido[4,3-b]indole[244-69-9], 28.8g(110mmol) of methyl 2-iodobenzoate[610-97 -9], 34.6g (250mmol) of potassium carbonate, 6.4g (100mmol) of copper powder and 100g of glass beads (3mm in diameter) in 300ml of DMF were stirred at 130°C for 24h. After cooling, the mixture was filtered through a short bed of celite, the bed was rinsed with DMF, and the DMF was removed in vacuo. The residue was dissolved in 500 ml of dichloromethane, and the solution was washed three times with 200 ml each of 0.5N sodium hydroxide solution and dried over magnesium sulfate. After removal of the dichloromethane under vacuum, the residue was recrystallized once from ethyl acetate and dried under vacuum (p=0.1 mbar, T=60°C). Yield: 19.3 g (64 mmol), 64%; Purity: 97% ( 1 H-NMR).
[0217] The following compounds are sim...
Embodiment 2
[0232] Example 2: 8,8-dimethyl-6,10-bis[1,1′; 3′,1″]terphenyl-5′-yl-8H-3,12b-diazabenzo[a ]acethracene
[0233] 2a) 6,10-Dibromo-8,8-dimethyl-H-3,12b-diazabenzo[a]acethracene
[0234]
[0235] 28.4g (100mmol) of 8,8-dimethyl-H-3,12b-diazabenzo[a]acethracene and 36.5g (205mmol) of N-bromosuccinimide in The mixture in 200ml DMF was stirred at 80°C for 16h. After cooling, 100 ml of ethanol were added, the precipitated solid was filtered off with suction, washed three times with 50 ml of ethanol and dried under vacuum (p=0.1 mbar, T=60° C.). Yield: 16.3 g (77 mmol), 77%; Purity: 98% ( 1 H-NMR).
[0236] 2b) 8,8-Dimethyl-6,10-bis[1,1′;3′,1″]terphenyl-5′-yl-8H-3,12b-diazabenzo[a]ester Anthracene
[0237]
[0238] 13.3g (30mmol) of 6,10-dibromo-8,8-dimethyl-H-3,12b-diazabenzo[a]acethracene, 24.7g (90mmol) of [1 , 1′: 3′, 1″-terphenyl]-5′-ylboronic acid [128388-54-5], 34.8g (180mmol) tripotassium phosphate, 67mg (0.3mmol) palladium(II), 548mg (1.8mmol) tri-o-tolylphosphi...
Embodiment 3
[0239] Example 3: Spiro[fluorenyl-9,8'-H-3,12b-diazabenzo[a]acethracene]
[0240] 3a) 3,12b-diazabenzo[a]acethren-8-one
[0241]
[0242]15.1 g (50 mmol) of methyl 2-pyrido[4,3-b]indol-5-ylbenzoate were suspended in a mixture of 200 ml of glacial acetic acid and 5 ml of concentrated sulfuric acid. The suspension was heated to reflux for 5 h, then the acetic acid was removed in vacuo, the residue was dissolved in 500 ml of dichloromethane and neutralized with 1N sodium hydroxide solution. The organic phase was separated off, dried over magnesium sulfate and evaporated under vacuum. The residue was dissolved in 30ml of ethyl acetate, and 100ml of ethanol was slowly added at high temperature. After cooling, the precipitated solid was filtered off with suction, washed three times with 50 ml of ethanol and dried under vacuum (p=0.1 mbar, T=60° C.). Yield: 10.5 g (39 mmol), 78%; Purity: 97% ( 1 H-NMR).
[0243] 3b) spiro[fluorenyl-9,8'-H-3,12b-diazabenzo[a]acethracene]
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com