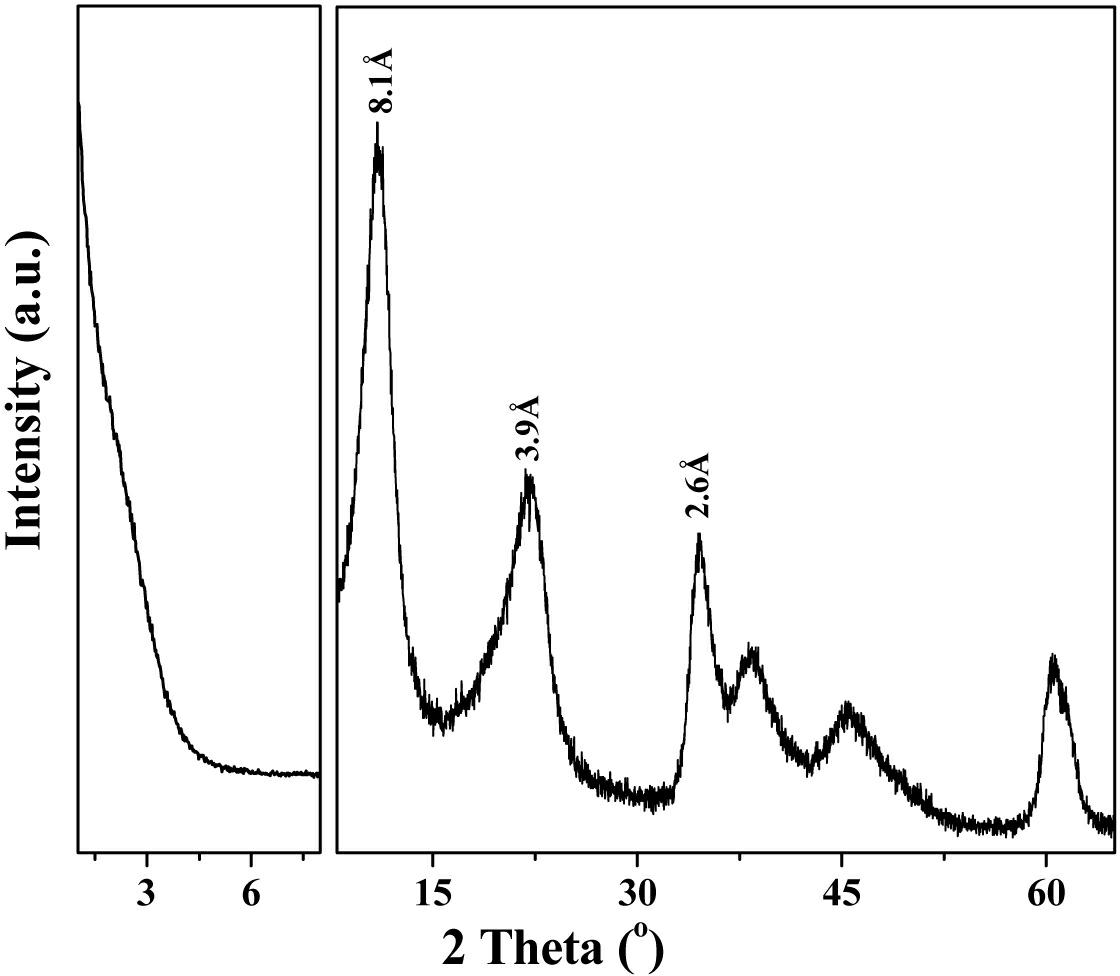
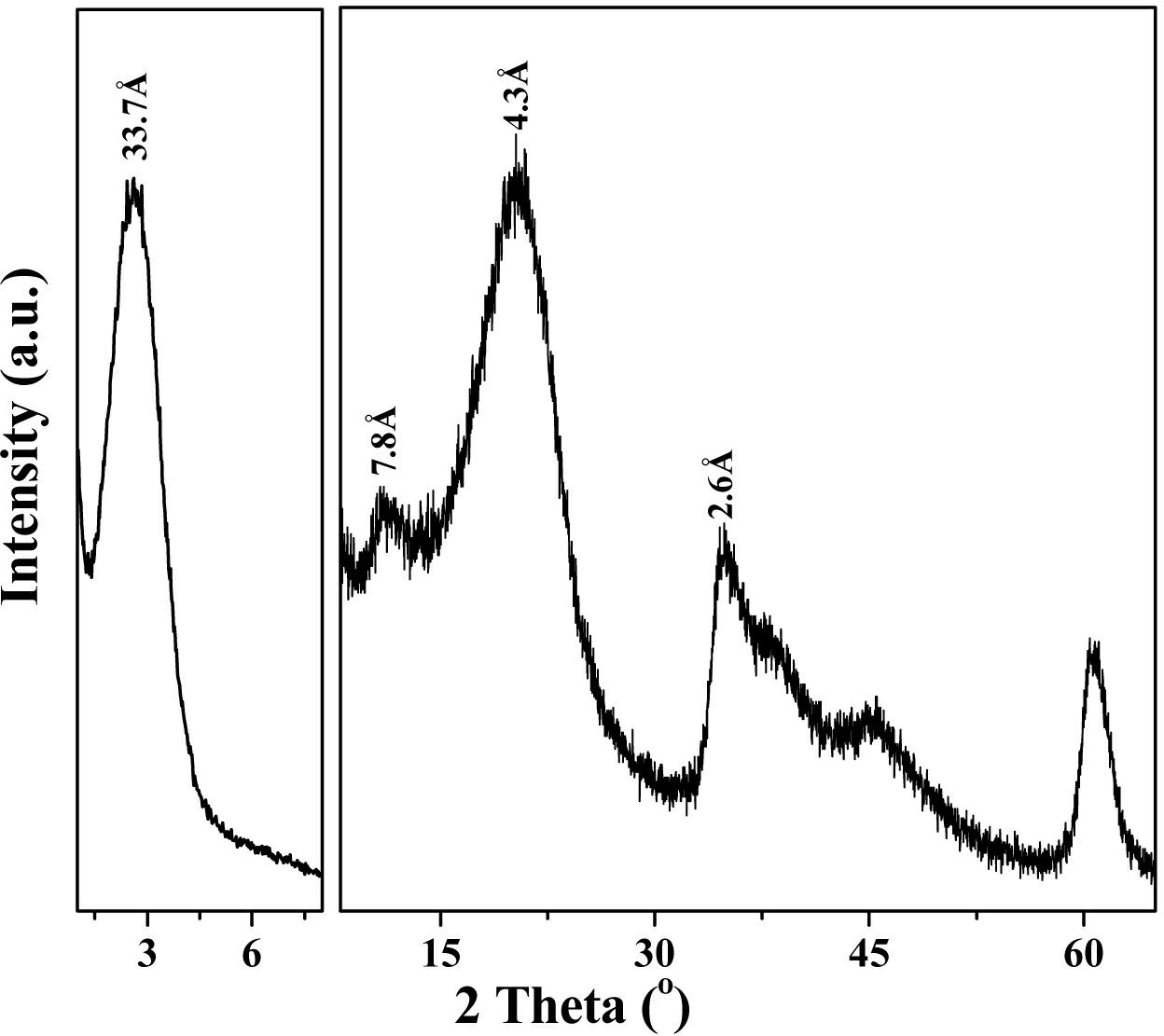
In-situ co-precipitation synthesis method for organic silane grafted hydrotalcite with controllable interlayer spacing
A technology of in-situ co-precipitation and synthesis method is applied in the field of preparation of interlayer controllable organosilane-grafted hydrotalcite, which can solve the problems of poor stability, poor stability and secondary pollution of composite materials, and achieves improved dispersibility and improved Dispersibility and homogeneity, the effect of improving affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Mg 2+ 、Al 3+ The hydrotalcite is the metal skeleton ion, the lauryl sodium sulfate is the interlayer spacing control agent, and the gamma-aminopropyl triethoxysilane is the organosilane grafted hydrotalcite as the graft modifier.
[0034] Weigh 19.2 g Mg(NO 3 ) 2 ·6H 2 O and 9.4 g Al(NO 3 ) 3 ?9H 2 O co-dissolves in 90 cm 3 deionized water (solution A). 8 g NaOH and 1.4 g sodium lauryl sulfate were dissolved in 50 cm 3 deionized water (solution B). 7.0cm 3 γ-Aminopropyltriethoxysilane dissolved in 50 cm 3 in ethanol (solution C). Add solutions A and B dropwise to 100 cm 3 In deionized water, when precipitation occurs, immediately add solution C dropwise. During the dropwise addition, the mixed solution was stirred rapidly, and the pH value of the mixed solution was controlled to be stable at about 10. After the dropwise addition was completed, stirring was continued for 6h. Wash with deionized water and ethanol three times each. The obtained precipitat...
Embodiment 2
[0037] Mg2+ 、Al 3+ The hydrotalcite is the metal skeleton ion, the lauryl sodium sulfate is the interlayer spacing control agent, and the gamma-aminopropyl triethoxysilane is the organosilane grafted hydrotalcite as the graft modifier.
[0038] Weigh 19.2 g Mg(NO 3 ) 2 ·6H 2 O and 9.4 g Al(NO 3 ) 3 ?9H 2 O co-dissolves in 90 cm 3 deionized water (solution A). 8 g NaOH and 2.2 g sodium lauryl sulfate were dissolved in 50 cm 3 deionized water (solution B). 5.0cm 3 γ-Aminopropyltriethoxysilane dissolved in 50 cm 3 in propanol (Solution C). Add solutions A and B dropwise to 100 cm 3 deionized water. When precipitation occurs, immediately add solution C dropwise. During the dropwise addition, the mixed solution was stirred rapidly, and the pH value of the mixed solution was controlled to be stable at about 10. After the dropwise addition was completed, stirring was continued for 6h. Wash with deionized water and propanol three times each. The obtained precipitate ...
Embodiment 3
[0040] Zn prepared by in situ co-precipitation 2+ 、Al 3+ The hydrotalcite is the metal skeleton ion, the lauryl sodium sulfate is the laminate spacing control agent, and the gamma-aminopropyl trimethoxysilane is the organosilane grafted hydrotalcite as the graft modifier.
[0041] Weigh 22.3 g Zn(NO 3 ) 2 ·6H 2 O and 9.4 g Al(NO 3 ) 3 ?9H 2 O co-dissolves in 90 cm 3 deionized water (solution A). 8 g NaOH and 4.3 g sodium lauryl sulfate were dissolved in 50 cm 3 deionized water (solution B). 5.0cm 3 γ-Aminopropyltrimethoxysilane dissolved in 50 cm 3 in isopropanol (Solution C). Add solutions A and B dropwise to 100 cm 3 deionized water. When precipitation occurs, immediately add solution C dropwise. During the dropwise addition, the mixed solution was stirred rapidly, and the pH value of the mixed solution was controlled to be stable at about 10. After the dropwise addition was completed, stirring was continued for 6h. Wash three times each with deionized wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com